
CHEBI:16595
| Name | 1D-myo-inositol 1,4,5-trisphosphate | 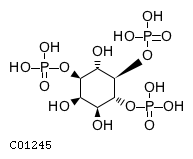 Download: mol | sdf |
| Synonyms | 01,4,5-insp3; 1d-myo-inositol 1,4,5-trisphosphate; 1d-myo-inositol 1,4,5-tris(phosphate); D-myo-inositol 1,4,5-trisphosphate; D-myo-inositol-1,4,5-triphosphate; Inositol 1,4,5-trisphosphate; Ins(1,4,5)p3; Insp3; Ip3; | |
| Definition | NA | |
| Molecular Weight (Exact mass) | 420.0956 (419.9624) | |
| Molecular Formula | C6H15O15P3 | |
| SMILES | O[C@@H]1[C@H](O)[C@@H](OP(O)(O)=O)[C@H](OP(O)(O)=O)[C@@H](O)[C@@H]1OP(O)(O)=O | |
| InChI | InChI=1S/C6H15O15P3/c7-1-2(8)5(20-23(13,14)15)6(21-24(16,17)18)3(9)4(1)19-22(10,11)12/h1-9H,(H2,10,11,12)(H2,13,14,15)(H2,16,17,18)/t1-,2+,3+,4-,5-,6-/m1/s1 | |
| InChI Key | MMWCIQZXVOZEGG-XJTPDSDZSA-N | |
| Crosslinking annotations | KEGG:C01245 | 3DMET:B01422 | CAS:85166-31-0 | ChEBI:16595 | ChEMBL:CHEMBL279107 | KNApSAcK:C00007460 | NIKKAJI:J260.291A | PDB-CCD:I3P | PubChem:4466 | |
| Pathway ID | Pathway Name | Pathway Description (KEGG) |
| map00562 | Inositol phosphate metabolism | NA |
| map01100 | Metabolic pathways | NA |
| map04010 | MAPK signaling pathway | The mitogen-activated protein kinase (MAPK) cascade is a highly conserved module that is involved in various cellular functions, including cell proliferation, differentiation and migration. Mammals express at least four distinctly regulated groups of MAPKs, extracellular signal-related kinases (ERK)-1/2, Jun amino-terminal kinases (JNK1/2/3), p38 proteins (p38alpha/beta/gamma/delta) and ERK5, that are activated by specific MAPKKs: MEK1/2 for ERK1/2, MKK3/6 for the p38, MKK4/7 (JNKK1/2) for the JNKs, and MEK5 for ERK5. Each MAPKK, however, can be activated by more than one MAPKKK, increasing the complexity and diversity of MAPK signalling. Presumably each MAPKKK confers responsiveness to distinct stimuli. For example, activation of ERK1/2 by growth factors depends on the MAPKKK c-Raf, but other MAPKKKs may activate ERK1/2 in response to pro-inflammatory stimuli. |
| map04012 | ErbB signaling pathway | The ErbB family of receptor tyrosine kinases (RTKs) couples binding of extracellular growth factor ligands to intracellular signaling pathways regulating diverse biologic responses, including proliferation, differentiation, cell motility, and survival. Ligand binding to the four closely related members of this RTK family -epidermal growth factor receptor (EGFR, also known as ErbB-1 or HER1), ErbB-2 (HER2), ErbB-3 (HER3), and ErbB-4 (HER4)-induces the formation of receptor homo- and heterodimers and the activation of the intrinsic kinase domain, resulting in phosphorylation on specific tyrosine residues (pY) within the cytoplasmic tail. Signaling effectors containing binding pockets for pY-containing peptides are recruited to activated receptors and induce the various signaling pathways. The Shc- and/or Grb2-activated mitogen-activated protein kinase (MAPK) pathway is a common target downstream of all ErbB receptors. Similarly, the phosphatidylinositol-3-kinase (PI-3K) pathway is directly or indirectly activated by most ErbBs. Several cytoplasmic docking proteins appear to be recruited by specific ErbB receptors and less exploited by others. These include the adaptors Crk, Nck, the phospholipase C gamma (PLCgamma), the intracellular tyrosine kinase Src, or the Cbl E3 ubiquitin protein ligase. |
| map04014 | Ras signaling pathway | The Ras proteins are GTPases that function as molecular switches for signaling pathways regulating cell proliferation, survival, growth, migration, differentiation or cytoskeletal dynamism. Ras proteins transduce signals from extracellular growth factors by cycling between inactive GDP-bound and active GTP-bound states. The exchange of GTP for GDP on RAS is regulated by guanine nucleotide exchange factors (GEFs) and GTPase-activating proteins (GAPs). Activated RAS (RAS-GTP) regulates multiple cellular functions through effectors including Raf, phosphatidylinositol 3-kinase (PI3K) and Ral guanine nucleotide-dissociation stimulator (RALGDS). |
| map04020 | Calcium signaling pathway | Ca2+ that enters the cell from the outside is a principal source of signal Ca2+. Entry of Ca2+ is driven by the presence of a large electrochemical gradient across the plasma membrane. Cells use this external source of signal Ca2+ by activating various entry channels with widely different properties. The voltage-operated channels (VOCs) are found in excitable cells and generate the rapid Ca2+ fluxes that control fast cellular processes. There are many other Ca2+-entry channels, such as the receptor-operated channels (ROCs), for example the NMDA (N-methyl-D-aspartate) receptors (NMDARs) that respond to glutamate. There also are second-messenger-operated channels (SMOCs) and store-operated channels (SOCs).The other principal source of Ca2+ for signalling is the internal stores that are located primarily in the endoplasmic/sarcoplasmic reticulum (ER/SR), in which inositol-1,4,5-trisphosphate receptors (IP3Rs) or ryanodine receptors (RYRs) regulate the release of Ca2+. The principal activator of these channels is Ca2+ itself and this process of Ca2+-induced Ca2+ release is central to the mechanism of Ca2+ signalling. Various second messengers or modulators also control the release of Ca2+. IP3, which is generated by pathways using different isoforms of phospholipase C (PLCbeta, delta, epsilon, gamma and zeta), regulates the IP3Rs. Cyclic ADP-ribose (cADPR) releases Ca2+ via RYRs. Nicotinic acid adenine dinucleotide phosphate (NAADP) may activate a distinct Ca2+ release mechanism on separate acidic Ca2+ stores. Ca2+ release via the NAADP-sensitive mechanism may also feedback onto either RYRs or IP3Rs. cADPR and NAADP are generated by CD38. This enzyme might be sensitive to the cellular metabolism, as ATP and NADH inhibit it.The influx of Ca2+ from the environment or release from internal stores causes a very rapid and dramatic increase in cytoplasmic calcium concentration, which has been widely exploited for signal transduction. Some proteins, such as troponin C (TnC) involved in muscle contraction, directly bind to and sense Ca2+. However, in other cases Ca2+ is sensed through intermediate calcium sensors such as calmodulin (CALM). |
| map04022 | cGMP-PKG signaling pathway | Cyclic GMP (cGMP) is the intracellular second messenger that mediates the action of nitric oxide (NO) and natriuretic peptides (NPs), regulating a broad array of physiologic processes. The elevated intracellular cGMP level exerts its physiological action through two forms of cGMP-dependent protein kinase (PKG), cGMP-regulated phosphodiesterases (PDE2, PDE3) and cGMP-gated cation channels, among which PKGs might be the primary mediator. PKG1 isoform-specific activation of established substrates leads to reduction of cytosolic calcium concentration and/or decrease in the sensitivity of myofilaments to Ca2+ (Ca2+-desensitization), resulting in smooth muscle relaxation. In cardiac myocyte, PKG directly phosphorylates a member of the transient potential receptor canonical channel family, TRPC6, suppressing this nonselective ion channel's Ca2+ conductance, G-alpha-q agonist-induced NFAT activation, and myocyte hypertrophic responses. PKG also opens mitochondrial ATP-sensitive K+ (mitoKATP) channels and subsequent release of ROS triggers cardioprotection. |
| map04024 | cAMP signaling pathway | cAMP is one of the most common and universal second messengers, and its formation is promoted by adenylyl cyclase (AC) activation after ligation of G protein-coupled receptors (GPCRs) by ligands including hormones, neurotransmitters, and other signaling molecules. cAMP regulates pivotal physiologic processes including metabolism, secretion, calcium homeostasis, muscle contraction, cell fate, and gene transcription. cAMP acts directly on three main targets: protein kinase A (PKA), the exchange protein activated by cAMP (Epac), and cyclic nucleotide-gated ion channels (CNGCs). PKA modulates, via phosphorylation, a number of cellular substrates, including transcription factors, ion channels, transporters, exchangers, intracellular Ca2+ -handling proteins, and the contractile machinery. Epac proteins function as guanine nucleotide exchange factors (GEFs) for both Rap1 and Rap2. Various effector proteins, including adaptor proteins implicated in modulation of the actin cytoskeleton, regulators of G proteins of the Rho family, and phospholipases, relay signaling downstream from Rap. |
| map04062 | Chemokine signaling pathway | Inflammatory immune response requires the recruitment of leukocytes to the site of inflammation upon foreign insult. Chemokines are small chemoattractant peptides that provide directional cues for the cell trafficking and thus are vital for protective host response. In addition, chemokines regulate plethora of biological processes of hematopoietic cells to lead cellular activation, differentiation and survival.The chemokine signal is transduced by chemokine receptors (G-protein coupled receptors) expressed on the immune cells. After receptor activation, the alpha- and beta-gamma-subunits of G protein dissociate to activate diverse downstream pathways resulting in cellular polarization and actin reorganization. Various members of small GTPases are involved in this process. Induction of nitric oxide and production of reactive oxygen species are as well regulated by chemokine signal via calcium mobilization and diacylglycerol production. |
| map04064 | NF-kappa B signaling pathway | Nuclear factor-kappa B (NF-kappa B) is the generic name of a family of transcription factors that function as dimers and regulate genes involved in immunity, inflammation and cell survival. There are several pathways leading to NF-kappa B-activation. The canonical pathway is induced by tumour necrosis factor-alpha (TNF-alpha), interleukin-1 (IL-1) or byproducts of bacterial and viral infections. This pathway relies on IKK- mediated IkappaB-alpha phosphorylation on Ser32 and 36, leading to its degradation, which allows the p50/p65 NF-kappa B dimer to enter the nucleus and activate gene transcription. Atypical pathways are IKK-independent and rely on phosphorylation of IkappaB-alpha on Tyr42 or on Ser residues in IkappaB-alpha PEST domain. The non-canonical pathway is triggered by particular members of the TNFR superfamily, such as lymphotoxin-beta (LT-beta) or BAFF. It involves NIK and IKK-alpha-mediated p100 phosphorylation and processing to p52, resulting in nuclear translocation of p52/RelB heterodimers. |
| map04066 | HIF-1 signaling pathway | Hypoxia-inducible factor 1 (HIF-1) is a transcription factor that functions as a master regulator of oxygen homeostasis. It consists of two subunits: an inducibly-expressed HIF-1alpha subunit and a constitutively-expressed HIF-1beta subunit. Under normoxia, HIF-1 alpha undergoes hydroxylation at specific prolyl residues which leads to an immediate ubiquitination and subsequent proteasomal degradation of the subunit. In contrast, under hypoxia, HIF-1 alpha subunit becomes stable and interacts with coactivators such as p300/CBP to modulate its transcriptional activity. Eventually, HIF-1 acts as a master regulator of numerous hypoxia-inducible genes under hypoxic conditions. The target genes of HIF-1 encode proteins that increase O2 delivery and mediate adaptive responses to O2 deprivation. Despite its name, HIF-1 is induced not only in response to reduced oxygen availability but also by other stimulants, such as nitric oxide, or various growth factors. |
| map04070 | Phosphatidylinositol signaling system | NA |
| map04071 | Sphingolipid signaling pathway | Sphingomyelin (SM) and its metabolic products are now known to have second messenger functions in a variety of cellular signaling pathways. Particularly, the sphingolipid metabolites, ceramide (Cer) and sphingosine-1-phosphate (S1P), have emerged as a new class of potent bioactive molecules. Ceramide can be generated de novo or by hydrolysis of membrane sphingomyelin by sphingomyelinase (SMase). Ceramide is subsequently metabolized by ceramidase to generate sphingosine (Sph) which in turn produces S1P through phosphorylation by sphingosine kinases 1 and 2 (SphK1, 2). Both ceramide and S1P regulate cellular responses to stress, with generally opposing effects. S1P functions as a growth and survival factor, acting as a ligand for a family of G protein-coupled receptors, whereas ceramide activates intrinsic and extrinsic apoptotic pathways through receptor-independent mechanisms. |
| map04072 | Phospholipase D signaling pathway | Phospholipase D (PLD) is an essential enzyme responsible for the production of the lipid second messenger phosphatidic acid (PA), which is involved in fundamental cellular processes, including membrane trafficking, actin cytoskeleton remodeling, cell proliferation and cell survival. PLD activity can be stimulated by a large number of cell surface receptors and is elaborately regulated by intracellular factors, including protein kinase C isoforms, small GTPases of the ARF, Rho and Ras families and the phosphoinositide, phosphatidylinositol 4,5-bisphosphate (PIP2). The PLD-produced PA activates signaling proteins and acts as a node within the membrane to which signaling proteins translocate. Several signaling proteins, including Raf-1 and mTOR, directly bind PA to mediate translocation or activation, respectively. |
| map04114 | Oocyte meiosis | During meiosis, a single round of DNA replication is followed by two rounds of chromosome segregation, called meiosis I and meiosis II. At meiosis I, homologous chromosomes recombine and then segregate to opposite poles, while the sister chromatids segregate from each other at meoisis II. In vertebrates, immature oocytes are arrested at the PI (prophase of meiosis I). The resumption of meiosis is stimulated by progesterone, which carries the oocyte through two consecutive M-phases (MI and MII) to a second arrest at MII. The key activity driving meiotic progression is the MPF (maturation-promoting factor), a heterodimer of CDC2 (cell division cycle 2 kinase) and cyclin B. In PI-arrested oocytes, MPF is initially inactive and is activated by the dual-specificity CDC25C phosphatase as the result of new synthesis of Mos induced by progesterone. MPF activation mediates the transition from the PI arrest to MI. The subsequent decrease in MPF levels, required to exit from MI into interkinesis, is induced by a negative feedback loop, where CDC2 brings about the activation of the APC (anaphase-promoting complex), which mediates destruction of cyclin B. Re-activation of MPF for MII requires re-accumulation of high levels of cyclin B as well as the inactivation of the APC by newly synthesized Emi2 and other components of the CSF (cytostatic factor), such as cyclin E or high levels of Mos. CSF antagonizes the ubiquitin ligase activity of the APC, preventing cyclin B destruction and meiotic exit until fertilization occurs. Fertilization triggers a transient increase in cytosolic free Ca2+, which leads to CSF inactivation and cyclin B destruction through the APC. Then eggs are released from MII into the first embryonic cell cycle. |
| map04261 | Adrenergic signaling in cardiomyocytes | Cardiac myocytes express at least six subtypes of adrenergic receptor (AR) which include three subtypes of beta-AR (beta-1, beta-2, beta-3) and three subtypes of the alpha-1-AR (alpha-1A, alpha-1B, and alpha-1C). In the human heart the beta-1-AR is the pre- dominate receptor. Acute sympathetic stimulation of cardiac beta-1-ARs induces positive inotropic and chronotropic effects, the most effective mechanism to acutely increase output of the heart, by coupling to Gs, formation of cAMP by adenylyl cyclase (AC), and PKA- dependent phosphorylation of various target proteins (e.g., ryanodine receptor [RyR]; phospholamban [PLB], troponin I [TnI], and the L-type Ca2+ channel [LTCC]). Chronic beta-1-AR stimulation is detrimental and induces cardiomyocyte hypertrophy and apoptosis. beta-2-AR coupled to Gs exerts a proapoptotic action as well as beta-1-AR, while beta-2-AR coupled to Gi exerts an antiapoptotic action. |
| map04270 | Vascular smooth muscle contraction | The vascular smooth muscle cell (VSMC) is a highly specialized cell whose principal function is contraction. On contraction, VSMCs shorten, thereby decreasing the diameter of a blood vessel to regulate the blood flow and pressure. The principal mechanisms that regulate the contractile state of VSMCs are changes in cytosolic Ca2+ concentration ([Ca2+]c). In response to vasoconstrictor stimuli, Ca2+ is mobilized from intracellular stores and/or the extracellular space to increase [Ca2+]c in VSMCs. The increase in [Ca2+]c, in turn, activates the Ca2+-CaM-MLCK pathway and stimulates MLC20 phosphorylation, leading to myosin-actin interactions and, hence, the development of contractile force. The sensitivity of contractile myofilaments or MLC20 phosphorylation to Ca2+ can be secondarily modulated by other signaling pathways. During receptor stimulation, the contractile force is greatly enhanced by the inhibition of myosin phosphatase. Rho/Rho kinase, PKC, and arachidonic acid have been proposed to play a pivotal role in this enhancement. The signaling events that mediate relaxation include the removal of a contractile agonist (passive relaxation) and activation of cyclic nucleotide-dependent signaling pathways in the continued presence of a contractile agonist (active relaxation). Active relaxation occurs through the inhibition of both Ca2+ mobilization and myofilament Ca2+ sensitivity in VSMCs. |
| map04370 | VEGF signaling pathway | There is now much evidence that VEGFR-2 is the major mediator of VEGF-driven responses in endothelial cells and it is considered to be a crucial signal transducer in both physiologic and pathologic angiogenesis. The binding of VEGF to VEGFR-2 leads to a cascade of different signaling pathways, resulting in the up-regulation of genes involved in mediating the proliferation and migration of endothelial cells and promoting their survival and vascular permeability. For example, the binding of VEGF to VEGFR-2 leads to dimerization of the receptor, followed by intracellular activation of the PLCgamma;PKC-Raf kinase-MEK-mitogen-activated protein kinase (MAPK) pathway and subsequent initiation of DNA synthesis and cell growth, whereas activation of the phosphatidylinositol 3' -kinase (PI3K)-Akt pathway leads to increased endothelial-cell survival. Activation of PI3K, FAK, and p38 MAPK is implicated in cell migration signaling. |
| map04371 | Apelin signaling pathway | Apelin is an endogenous peptide capable of binding the apelin receptor (APJ), which was originally described as an orphan G-protein-coupled receptor. Apelin and APJ are widely expressed in various tissues and organ systems. They are implicated in different key physiological processes such as angiogenesis, cardiovascular functions, cell proliferation and energy metabolism regulation. On the other hand, this ligand receptor couple is also involved in several pathologies including diabetes, obesity, cardiovascular disease and cancer. |
| map04380 | Osteoclast differentiation | The osteoclasts, multinucleared cells originating from the hematopoietic monocyte-macrophage lineage, are responsible for bone resorption. Osteoclastogenesis is mainly regulated by signaling pathways activated by RANK and immune receptors, whose ligands are expressed on the surface of osteoblasts. Signaling from RANK changes gene expression patterns through transcription factors like NFATc1 and characterizes the active osteoclast. |
| map04540 | Gap junction | Gap junctions contain intercellular channels that allow direct communication between the cytosolic compartments of adjacent cells. Each gap junction channel is formed by docking of two 'hemichannels', each containing six connexins, contributed by each neighboring cell. These channels permit the direct transfer of small molecules including ions, amino acids, nucleotides, second messengers and other metabolites between adjacent cells. Gap junctional communication is essential for many physiological events, including embryonic development, electrical coupling, metabolic transport, apoptosis, and tissue homeostasis. Communication through Gap Junction is sensitive to a variety of stimuli, including changes in the level of intracellular Ca2+, pH, transjunctional applied voltage and phosphorylation/dephosphorylation processes. This figure represents the possible activation routes of different protein kinases involved in Cx43 and Cx36 phosphorylation. |
| map04611 | Platelet activation | Platelets play a key and beneficial role for primary hemostasis on the disruption of the integrity of vessel wall. Platelet adhesion and activation at sites of vascular wall injury is initiated by adhesion to adhesive macromolecules, such as collagen and von Willebrand factor (vWF), or by soluble platelet agonists, such as ADP, thrombin, and thromboxane A2. Different receptors are stimulated by various agonists, almost converging in increasing intracellular Ca2+ concentration that stimulate platelet shape change and granule secretion and ultimately induce the inside-out signaling process leading to activation of the ligand-binding function of integrin alpha IIb beta 3. Binding of alpha IIb beta 3 to its ligands, mainly fibrinogen, mediates platelet adhesion and aggregation and triggers outside-in signaling, resulting in platelet spreading, additional granule secretion, stabilization of platelet adhesion and aggregation, and clot retraction. |
| map04621 | NOD-like receptor signaling pathway | Specific families of pattern recognition receptors are responsible for detecting various pathogens and generating innate immune responses. The intracellular NOD-like receptor (NLR) family contains more than 20 members in mammals and plays a pivotal role in the recognition of intracellular ligands. NOD1 and NOD2, two prototypic NLRs, sense the cytosolic presence of the bacterial peptidoglycan fragments that escaped from endosomal compartments, driving the activation of NF-{kappa}B and MAPK, cytokine production and apoptosis. On the other hand, a different set of NLRs induces caspase-1 activation through the assembly of multiprotein complexes called inflammasomes. The activated of caspase-1 regulates maturation of the pro-inflammatory cytokines IL-1B, IL-18 and drives pyroptosis. |
| map04625 | C-type lectin receptor signaling pathway | C-type lectin receptors (CLRs) are a large superfamily of proteins characterized by the presence of one or more C-type lectin-like domains (CTLDs). CLRs function as pattern-recognition receptors (PRRs) for pathogen-derived ligands in dendric cells, macrophages, neutrophils, etc., such as Dectin-1 and Dectin-2 for recognition of fungi-derived B-glucan and high mannose-type carbohydrates. Upon ligand binding, CLRs stimulate intracellular signaling cascades that induce the production of inflammatory cytokines and chemokines, consequently triggering innate and adaptive immunity to pathogens. |
| map04650 | Natural killer cell mediated cytotoxicity | Natural killer (NK) cells are lymphocytes of the innate immune system that are involved in early defenses against both allogeneic (nonself) cells and autologous cells undergoing various forms of stress, such as infection with viruses, bacteria, or parasites or malignant transformation. Although NK cells do not express classical antigen receptors of the immunoglobulin gene family, such as the antibodies produced by B cells or the T cell receptor expressed by T cells, they are equipped with various receptors whose engagement allows them to discriminate between target and nontarget cells. Activating receptors bind ligands on the target cell surface and trigger NK cell activation and target cell lysis. However Inhibitory receptors recognize MHC class I molecules (HLA) and inhibit killing by NK cells by overruling the actions of the activating receptors. This inhibitory signal is lost when the target cells do not express MHC class I and perhaps also in cells infected with virus, which might inhibit MHC class I exprssion or alter its conformation. The mechanism of NK cell killing is the same as that used by the cytotoxic T cells generated in an adaptive immune response; cytotoxic granules are released onto the surface of the bound target cell, and the effector proteins they contain penetrate the cell membrane and induce programmed cell death. |
| map04658 | Th1 and Th2 cell differentiation | Immunity to different classes of microorganisms is orchestrated by separate lineages of effector T helper (TH)-cells, which differentiate from naive CD4+ precursor cells in response to cues provided by antigen presenting cells (APC) and include T helper type 1 (Th1) and Th2. Th1 cells are characterized by the transcription factor T-bet and signal transducer and activator of transcription (STAT) 4, and the production of IFN-gamma. These cells stimulate strong cell-mediated immune responses, particularly against intracellular pathogens. On the other hand, transcription factors like GATA-3 and STAT6 drive the generation of Th2 cells that produce IL-4, IL-5 and IL-13 and are necessary for inducing the humoral response to combat parasitic helminths (type 2 immunity) and isotype switching to IgG1 and IgE. The balance between Th1/Th2 subsets determines the susceptibility to disease states, where the improper development of Th2 cells can lead to allergy, while an overactive Th1 response can lead to autoimmunity. |
| map04659 | Th17 cell differentiation | Interleukin (IL)-17-producing helper T (Th17) cells serve as a subset of CD4+ T cells involved in epithelial cell- and neutrophil mediated immune responses against extracellular microbes and in the pathogenesis of autoimmune diseases. In vivo, Th17 differentiation requires antigen presentation and co-stimulation, and activation of antigen presenting-cells (APCs) to produce TGF-beta, IL-6, IL-1, IL-23 and IL-21. This initial activation results in the activation and up-regulation of STAT3, ROR(gamma)t and other transcriptional factors in CD4+ T cells, which bind to the promoter regions of the IL-17, IL-21 and IL-22 genes and induce IL-17, IL-21 and IL-22. In contrast, the differentiation of Th17 cells and their IL-17 expression are negatively regulated by IL-2, Th2 cytokine IL-4, IL-27 and Th1 cytokine IFN-gamma through STAT5, STAT6 and STAT1 activation, respectively. Retinoid acid and the combination of IL-2 and TGF-beta upregulate Foxp3, which also downregulates cytokines like IL-17 and IL-21. The inhibition of Th17 differentiation may serve as a protective strategy to 'fine-tune' the expression IL-17 so it does not cause excessive inflammation. Thus, balanced differentiation of Th cells is crucial for immunity and host protection. |
| map04660 | T cell receptor signaling pathway | Activation of T lymphocytes is a key event for an efficient response of the immune system. It requires the involvement of the T-cell receptor (TCR) as well as costimulatory molecules such as CD28. Engagement of these receptors through the interaction with a foreign antigen associated with major histocompatibility complex molecules and CD28 counter-receptors B7.1/B7.2, respectively, results in a series of signaling cascades. These cascades comprise an array of protein-tyrosine kinases, phosphatases, GTP-binding proteins and adaptor proteins that regulate generic and specialised functions, leading to T-cell proliferation, cytokine production and differentiation into effector cells. |
| map04662 | B cell receptor signaling pathway | B cells are an important component of adaptive immunity. They produce and secrete millions of different antibody molecules, each of which recognizes a different (foreign) antigen. The B cell receptor (BCR) is an integral membrane protein complex that is composed of two immunoglobulin (Ig) heavy chains, two Ig light chains and two heterodimers of Ig-alpha and Ig-beta. After BCR ligation by antigen, three main protein tyrosine kinases (PTKs) -the SRC-family kinase LYN, SYK and the TEC-family kinase BTK- are activated. Phosphatidylinositol 3-kinase (PI3K) and phospholipase C-gamma 2 (PLC-gamma 2) are important downstream effectors of BCR signalling. This signalling ultimately results in the expression of immediate early genes that further activate the expression of other genes involved in B cell proliferation, differentiation and Ig production as well as other processes. |
| map04664 | Fc epsilon RI signaling pathway | Fc epsilon RI-mediated signaling pathways in mast cells are initiated by the interaction of antigen (Ag) with IgE bound to the extracellular domain of the alpha chain of Fc epsilon RI. The activation pathways are regulated both positively and negatively by the interactions of numerous signaling molecules. Mast cells that are thus activated release preformed granules which contain biogenic amines (especially histamines) and proteoglycans (especially heparin). The activation of phospholipase A2 causes the release of membrane lipids followed by development of lipid mediators such as leukotrienes (LTC4, LTD4 and LTE4) and prostaglandins (especially PDG2). There is also secretion of cytokines, the most important of which are TNF-alpha, IL-4 and IL-5. These mediators and cytokines contribute to inflammatory responses. |
| map04666 | Fc gamma R-mediated phagocytosis | Phagocytosis plays an essential role in host-defense mechanisms through the uptake and destruction of infectious pathogens. Specialized cell types including macrophages, neutrophils, and monocytes take part in this process in higher organisms. After opsonization with antibodies (IgG), foreign extracellular materials are recognized by Fc gamma receptors. Cross-linking of Fc gamma receptors initiates a variety of signals mediated by tyrosine phosphorylation of multiple proteins, which lead through the actin cytoskeleton rearrangements and membrane remodeling to the formation of phagosomes. Nascent phagosomes undergo a process of maturation that involves fusion with lysosomes. The acquisition of lysosomal proteases and release of reactive oxygen species are crucial for digestion of engulfed materials in phagosomes. |
| map04720 | Long-term potentiation | Hippocampal long-term potentiation (LTP), a long-lasting increase in synaptic efficacy, is the molecular basis for learning and memory. Tetanic stimulation of afferents in the CA1 region of the hippocampus induces glutamate release and activation of glutamate receptors in dendritic spines. A large increase in [Ca2+]i resulting from influx through NMDA receptors leads to constitutive activation of CaM kinase II (CaM KII) . Constitutively active CaM kinase II phosphorylates AMPA receptors, resulting in potentiation of the ionic conductance of AMPA receptors. Early-phase LTP (E-LTP) expression is due, in part, to this phosphorylation of the AMPA receptor. It is hypothesized that postsynaptic Ca2+ increases generated through NMDA receptors activate several signal transduction pathways including the Erk/MAP kinase and cAMP regulatory pathways. The convergence of these pathways at the level of the CREB/CRE transcriptional pathway may increase expression of a family of genes required for late-phase LTP (L-LTP). |
| map04722 | Neurotrophin signaling pathway | Neurotrophins are a family of trophic factors involved in differentiation and survival of neural cells. The neurotrophin family consists of nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), neurotrophin 3 (NT-3), and neurotrophin 4 (NT-4). Neurotrophins exert their functions through engagement of Trk tyrosine kinase receptors or p75 neurotrophin receptor (p75NTR). Neurotrophin/Trk signaling is regulated by connecting a variety of intracellular signaling cascades, which include MAPK pathway, PI-3 kinase pathway, and PLC pathway, transmitting positive signals like enhanced survival and growth. On the other hand, p75NTR transmits both positive and nagative signals. These signals play an important role for neural development and additional higher-order activities such as learning and memory. |
| map04723 | Retrograde endocannabinoid signaling | Endogenous cannabinoids (endocannabinoids) serve as retrograde messengers at synapses in various regions of the brain. The family of endocannabinoids includes at least five derivatives of arachidonic acid; the two best characterized are arachydonoyl ethanolamide (anandamide, AEA) and 2-arachydonoil glycerol (2AG). They are released from postsynaptic neurons upon postsynaptic depolarization and/or receptor activation. The released endocannabinoids then activate the CB1 receptors (CB1R) at presynaptic terminals and suppress the release of inhibitory transmitter GABA (depolarization-induced suppression of inhibition, DSI) or excitatory transmitter glutamate (depolarization-induced suppression of excitation, DSE) by inhibiting Ca2+ channels. Besides the well-known expression of the CB1R in the plasma membrane, this receptor is also present in mitochondrial membranes, where it reduces the mitochondrial respiration and contributes to DSI. Whereas DSI and DSE result in short-term synaptic plasticity, endocannabinoids also mediate long-term synaptic changes (eCB-LTD). Persistent activation of CB1 receptors over a period of minutes triggers eCB-LTD by a RIM1alpha-dependent mechanism. |
| map04724 | Glutamatergic synapse | Glutamate is the major excitatory neurotransmitter in the mammalian central nervous system(CNS). Glutamate is packaged into synaptic vesicles in the presynaptic terminal. Once released into the synaptic cleft, glutamate acts on postsynaptic ionotropic glutamate receptors (iGluRs) to mediate fast excitatory synaptic transmission. Glutamate can also act on metabotropic glutamate receptors (mGluRs) and exert a variety of modulatory effects through their coupling to G proteins and the subsequent recruitment of second messenger systems. Presynaptically localized Group II and Group III mGluRs are thought to represent the classical inhibitory autoreceptor mechanism that suppresses excess glutamate release. After its action on these receptors, glutamate can be removed from the synaptic cleft by EAATs located either on the presynaptic terminal, neighboring glial cells, or the postsynaptic neuron. In glia, glutamate is converted to glutamine, which is then transported back to the presynaptic terminal and converted back to glutamate. |
| map04725 | Cholinergic synapse | Acetylcholine (ACh) is a neurotransmitter widely distributed in the central (and also peripheral, autonomic and enteric) nervous system (CNS). In the CNS, ACh facilitates many functions, such as learning, memory, attention and motor control. When released in the synaptic cleft, ACh binds to two distinct types of receptors: Ionotropic nicotinic acetylcholine receptors (nAChR) and metabotropic muscarinic acetylcholine receptors (mAChRs). The activation of nAChR by ACh leads to the rapid influx of Na+ and Ca2+ and subsequent cellular depolarization. Activation of mAChRs is relatively slow (milliseconds to seconds) and, depending on the subtypes present (M1-M5), they directly alter cellular homeostasis of phospholipase C, inositol trisphosphate, cAMP, and free calcium. In the cleft, ACh may also be hydrolyzed by acetylcholinesterase (AChE) into choline and acetate. The choline derived from ACh hydrolysis is recovered by a presynaptic high-affinity choline transporter (CHT). |
| map04726 | Serotonergic synapse | Serotonin (5-Hydroxytryptamine, 5-HT) is a monoamine neurotransmitter that plays important roles in physiological functions such as learning and memory, emotion, sleep, pain, motor function and endocrine secretion, as well as in pathological states including abnormal mood and cognition. Once released from presynaptic axonal terminals, 5-HT binds to receptors, which have been divided into 7 subfamilies on the basis of conserved structures and signaling mechanisms. These families include the ionotropic 5-HT3 receptors and G-protein-coupled 5-HT receptors, the 5-HT1 (Gi /Go -coupled), 5-HT2(Gq-coupled), 5-HT4/6/7 (Gs-coupled) and 5-HT5 receptors. Presynaptically localized 5-HT1B receptors are thought to be the autoreceptors that suppress excess 5-HT release. 5-HT's actions are terminated by transporter- mediated reuptake into neurons, leading to catabolism by monoamine oxidase. |
| map04728 | Dopaminergic synapse | Dopamine (DA) is an important and prototypical slow neurotransmitter in the mammalian brain, where it controls a variety of functions including locomotor activity, motivation and reward, learning and memory, and endocrine regulation. Once released from presynaptic axonal terminals, DA interacts with at least five receptor subtypes in the central nervous system (CNS), which have been divided into two groups: the D1-like receptors (D1Rs), comprising D1 and D5 receptors, both positively coupled to adenylyl cyclase and cAMP production, and the D2-like receptors (D2Rs), comprising D2, D3, and D4 receptors, whose activation results in inhibition of adenylyl cyclase and suppression of cAMP production. In addition, D1Rs and D2Rs modulate intracellular Ca2+ levels and a number of Ca2+ -dependent intracellular signaling processes. Through diverse cAMP- and Ca2+-dependent and - independent mechanisms, DA influences neuronal activity, synaptic plasticity, and behavior. Presynaptically localized D2Rs regulate synthesis and release of DA as the main autoreceptor of the dopaminergic system. |
| map04730 | Long-term depression | Cerebellar long-term depression (LTD), thought to be a molecular and cellular basis for cerebellar learning, is a process involving a decrease in the synaptic strength between parallel fiber (PF) and Purkinje cells (PCs) induced by the conjunctive activation of PFs and climbing fiber (CF). Multiple signal transduction pathways have been shown to be involved in this process. Activation of PFs terminating on spines in dendritic branchlets leads to glutamate release and activation of both AMPA and mGluRs. Activation of CFs, which make multiple synaptic contacts on proximal dendrites, also via AMPA receptors, opens voltage-gated calcium channels (VGCCs) and causes a generalized influx of calcium. These cellular signals, generated from two different synaptic origins, trigger a cascade of events culminating in a phosphorylation-dependent, long-term reduction in AMPA receptor sensitivity at the PF-PC synapse. This may take place either through receptor internalization and/or through receptor desensitization. |
| map04742 | Taste transduction | Five basic tastes are recognized by humans and most other animals - bitter, sweet, sour, salty and umami. In vertebrates, taste stimuli are detected by taste receptor cells (TRCs). At least three distinct cell types are found in mammalian taste buds : type I cells, type II cells, and type III cells. Type I cells express epithelial sodium channel (ENaC) and are considered to be the major mediator of perception of low salt. In type II cells, transduction of bitter, sweet and umami is mediated by a canonical PLC-beta/IP3-signaling cascade, which culminates in the opening of the TRPM5 ion channel. This produces a depolarization that may allow CALMH1 channels to open and release ATP, which serves as a neurotransmitter to activate closely associated nerve afferents expressing P2X2, P2X3 receptors and adjacent type III cells expressing P2Y4 receptors. Type II taste cells also secrete acetylcholine (ACh) that appears to stimulate muscarinic receptors, specifically M3, on the same or neighboring Type II cells. This muscarinic feedback augments taste-evoked release of ATP. In type III cells, sour taste is initiated when protons enter through apically located proton-selective ion channels: polycystic kidney disease 2-like 1 protein (PKD2L1) and polycystic kidney disease 1-like 3 protein (PKD1L3) channels. Weak acids may also activate sour cells by penetrating the cell membrane and leading to closure of resting K+ channels and membrane depolarization. Further, voltage-gated Ca2+ channels are activated and release vesicular serotonin (5-HT), norepinephrine (NE) and gamma-aminobutyric acid (GABA). 5-HT and GABA provide negative paracrine feedback onto receptor cells by activating 5-HT1A and GABAA, GABAB receptors, respectively. 5-HT also functions as a transmitter between presynaptic cells and the sensory afferent. |
| map04745 | Phototransduction - fly | Phototransduction is the process that converts the signal of light (photons) into a change of membrane potential in photoreceptor cells. Drosophila visual signaling is initiated with the activation of rhodopsin by light. Rhodopsin is composed of a protein, opsin, covalently linked to a chromophore, 3-hydroxy-11-cis-retinal. Upon absorption of a light photon the chromophore is isomerized from the 11-cis to the all-trans configuration which induces a structural change that activates the opsin. This photoconversion activates heterotrimeric Gq protein via GTP-GDP exchange, releasing the G(alpha)q subunit. G(alpha)q activates PLC, generating InsP3 and DAG from PIP2. DAG may further release polyunsaturated fatty acids (PUFAs) via action of DAG lipase. This reaction leads to the opening of cation-selective channels (trp and trpl genes) and causes the depolarization of the photoreceptor cells. The transduction proteins rhodopsin, G(alpha)q, PLC, PKC, TRP, and TRPL are coordinated by a polymer of INAD scaffold proteins. |
| map04750 | Inflammatory mediator regulation of TRP channels | The TRP channels that exhibit a unique response to temperature have been given the name thermo-TRPs. Among all thermo- TRP channels, TRPV1-4, TRPM8, and TRPA1 are expressed in subsets of nociceptive dorsal root ganglion (DRG) neuron cell bodies including their peripheral and central projections. These channels can be modulated indirectly by inflammatory mediators such as PGE2, bradykinin, ATP, NGF, and proinflammatory cytokines that are generated during tissue injury. While the noxious heat receptor TRPV1 is sensitized (that is, their excitability can be increased) by post-translational modifications upon activation of G-protein coupled receptors (GPCRs) or tyrosine kinase receptors, the receptors for inflammatory mediators, the same action appears to mainly desensitize TRPM8, the main somatic innocuous cold sensor. This aforementioned sensitization could allow the receptor to become active at body temperature, so it not only contributes toward thermal hypersensitivity but also is possibly a substrate for ongoing persistent pain. |
| map04911 | Insulin secretion | Pancreatic beta cells are specialised endocrine cells that continuously sense the levels of blood sugar and other fuels and, in response, secrete insulin to maintain normal fuel homeostasis. Glucose-induced insulin secretion and its potentiation constitute the principal mechanism of insulin release. Glucose is transported by the glucose transporter (GLUT) into the pancreatic beta-cell. Metabolism of glucose generates ATP, which inhibits ATP-sensitive K+ channels and causes voltage-dependent Ca2+ influx. Elevation of [Ca2+]i triggers exocytotic release of insulin granules. Insulin secretion is further regulated by several hormones and neurotransmitters. Peptide hormones, such as glucagon-like peptide 1 (GLP-1), increase cAMP levels and thereby potentiate insulin secretion via the combined action of PKA and Epac2. Achetylcholine (ACh), a major parasympathetic neurotransmitter, binds to Gq-coupled receptors and activates phospholipase C- (PLC-), and the stimulatory effects involve activation of protein kinase C (PKC), which stimulates exocytosis. In addition, ACh mobilizes intracellular Ca2+ by activation of IP3 receptors. |
| map04912 | GnRH signaling pathway | Gonadotropin-releasing hormone (GnRH) secretion from the hypothalamus acts upon its receptor in the anterior pituitary to regulate the production and release of the gonadotropins, LH and FSH. The GnRHR is coupled to Gq/11 proteins to activate phospholipase C which transmits its signal to diacylglycerol (DAG) and inositol 1,4,5-trisphosphate (IP3). DAG activates the intracellular protein kinase C (PKC) pathway and IP3 stimulates release of intracellular calcium. In addition to the classical Gq/11, coupling of Gs is occasionally observed in a cell-specific fashion. Signaling downstream of protein kinase C (PKC) leads to transactivation of the epidermal growth factor (EGF) receptor and activation of mitogen-activated protein kinases (MAPKs), including extracellular-signal-regulated kinase (ERK), Jun N-terminal kinase (JNK) and p38 MAPK. Active MAPKs translocate to the nucleus, resulting in activation of transcription factors and rapid induction of early genes. |
| map04915 | Estrogen signaling pathway | Estrogens are steroid hormones that regulate a plethora of physiological processes in mammals, including reproduction, cardiovascular protection, bone integrity, cellular homeostasis, and behavior. Estrogen mediates its cellular actions through two signaling pathways classified as nuclear-initiated steroid signaling and membrane-initiated steroid signaling. In the nuclear pathway, estrogen binds either ERalpha or ERbeta, which in turn translocates to the nucleus, binds DNA at ERE elements and activates the expression of ERE-dependent genes. In membrane pathway, Estrogen can exert its actions through a subpopulation of ER at the plasma membrane (mER) or novel G-protein coupled E2 receptors (GPER). Upon activation of these receptors various signaling pathways (i.e. Ca2+, cAMP, protein kinase cascades) are rapidly activated and ultimately influence downstream transcription factors. |
| map04916 | Melanogenesis | Cutaneous melanin pigment plays a critical role in camouflage, mimicry, social communication, and protection against harmful effects of solar radiation. Melanogenesis is under complex regulatory control by multiple agents. The most important positive regulator of melanogenesis is the MC1 receptor with its ligands melanocortic peptides. MC1R activates the cyclic AMP (cAMP) response-element binding protein (CREB). Increased expression of MITF and its activation by phosphorylation (P) stimulate the transcription of tyrosinase (TYR), tyrosinase-related protein 1 (TYRP1), and dopachrome tautomerase (DCT), which produce melanin. Melanin synthesis takes place within specialized intracellular organelles named melanosomes. Melanin-containing melanosomes then move from the perinuclear region to the dendrite tips and are transferred to keratinocytes by a still not well-characterized mechanism. |
| map04918 | Thyroid hormone synthesis | Thyroid hormones triiodothyronine (T3) and thyroxine (T4) are essential for normal development, growth and metabolic homeostasis in all vertebrates, and synthesized in the thyroid gland. The functional unit of the thyroid gland is the follicle, delimited by a monolayer of thyrocytes. Polarized thyrocytes surround the follicular lumen; with their basal and apical surfaces facing the bloodstream and the lumen, respectively. To synthesize thyroid hormones, thyrocytes take up iodide at their basal side and concentrate it into the lumen. They also secrete in this lumen the specialized protein thyroglobulin (TG) which serves as a store for the hormones. In the follicular lumen oxidation of iodine, iodination of tyrosines (MIT, 3-monoiodotyrosine; DIT, 3,5-diiodotyrosine) and coupling of iodotyrosines takes place on tyrosine residues in TG, resulting in T3 and T4 synthesis. Iodinated TG is resorbed through the apical membrane and degraded to form T3/T4 in lysosomes; the T3/T4 is then secreted through the basal membrane. |
| map04919 | Thyroid hormone signaling pathway | The thyroid hormones (THs) are important regulators of growth, development and metabolism. The action of TH is mainly mediated by T3 (3,5,3'-triiodo-L-thyronine). Thyroid hormones, L-thyroxine (T4) and T3 enter the cell through transporter proteins. Although the major form of TH in the blood is T4, it is converted to the more active hormone T3 within cells. T3 binds to nuclear thyroid hormone receptors (TRs), which functions as a ligand-dependent transcription factor and controls the expression of target genes (genomic action). Nongenomic mechanisms of action is initiated at the integrin receptor. The plasma membrane alpha(v)beta(3)-integrin has distinct binding sites for T3 and T4. One binding site binds only T3 and activates the phosphatidylinositol 3-kinase (PI3K) pathway. The other binding site binds both T3 and T4 and activates the ERK1/2 MAP kinase pathway. |
| map04921 | Oxytocin signaling pathway | Oxytocin (OT) is a nonapeptide synthesized by the magno-cellular neurons located in the supraoptic (SON) and paraventricular (PVN) nuclei of the hypothalamus. It exerts a wide variety of central and peripheral effects. However, its best-known and most well-established roles are stimulation of uterine contractions during parturition and milk release during lactation. Oxytocin also influences cardiovascular regulation and various social behaviors. The actions of OT are all mediated by one type of OT receptor (OTR). This is a transmembrane receptor belonging to the G-protein-coupled receptor superfamily. The main signaling pathway is the Gq/PLC/Ins3 pathway, but the MAPK and the RhoA/Rho kinase pathways are also activated, contributing to increased prostaglandin production and direct contractile effect on myometrial cells. In the cardiovascular system, OTR is associated with the ANP-cGMP and NO-cGMP pathways, which reduce the force and rate of contraction and increase vasodilatation. |
| map04922 | Glucagon signaling pathway | Glucagon is conventionally regarded as a counterregulatory hormone for insulin and plays a critical anti-hypoglycemic role by maintaining glucose homeostasis in both animals and humans. To increase blood glucose, glucagon promotes hepatic glucose output by increasing glycogenolysis and gluconeogenesis and by decreasing glycogenesis and glycolysis in a concerted fashion via multiple mechanisms. Glucagon also stimulates hepatic mitochondrial beta-oxidation to supply energy for glucose production. Glucagon performs its main effect via activation of adenylate cyclase. The adenylate-cyclase-derived cAMP activates protein kinase A (PKA), which then phosphorylates downstream targets, such as cAMP response element binding protein (CREB) and the bifunctional enzyme 6-phosphofructo-2-kinase/ fructose-2,6-bisphosphatase (one of the isoforms being PFK/FBPase 1, encoded by PFKFB1). |
| map04924 | Renin secretion | The aspartyl-protease renin is the key regulator of the renin-angiotensin-aldosterone system, which is critically involved in extracellular fluid volume and blood pressure homeostasis of the body. Renin is synthesized, stored in, and released into circulation by the juxtaglomerular (JG) cells of the kidney. Secretion of renin from JG cells at the organ level is controlled by the four main mechanisms: the sympathetic nervous system, the local JG apparatus baroreflex, the macula densa mechanism, and several hormones acting locally within the JG apparatus. Renin secretion at the level of renal JG cells appears to be controlled mainly by classic second messengers, namely cAMP, cGMP, and free cytosolic calcium concentration. While cAMP generally stimulates renin release and the intracellular calcium concentration suppresses the exocytosis of renin, the effects of cGMP in the regulation of the renin system are more complex as it both may stimulate or inhibit renin release. |
| map04925 | Aldosterone synthesis and secretion | Aldosterone is a steroid hormone synthesized in and secreted from the outer layer of the adrenal cortex, the zona glomerulosa. Aldosterone plays an important role in the regulation of systemic blood pressure through the absorption of sodium and water. Angiotensin II (Ang II), potassium (K+) and adrenocorticotropin (ACTH) are the main extracellular stimuli which regulate aldosterone secretion. These physiological agonists all converge on two major intracellular signaling pathways: calcium (Ca2+) mobilization and an increase in cAMP production. The increase in cytosolic calcium levels activates calcium/calmodulin- dependent protein kinases (CaMK), and the increased cAMP levels stimulate the activity of cAMP-dependent protein kinase, or protein kinase A (PKA). The activated CaMK, and possibly PKA, activates transcription factors (NURR1 and NGF1B, CREB) to induce StAR and CYP11B2 expression, the early and late rate- limiting steps in aldosterone biosynthesis, respectively, thereby stimulating aldosterone secretion. |
| map04926 | Relaxin signaling pathway | Human relaxin-2 (relaxin), originally identified as a peptidic hormone of pregnancy, is now known to exert a range of pleiotropic effects including vasodilatory, anti-fibrotic and angiogenic effects in both males and females. It belongs to the so-called relaxin peptide family which includes the insulin-like peptides INSL3 and INSL5, and relaxin-3 (H3) as well as relaxin. INSL3 has clearly defined specialist roles in male and female reproduction, relaxin-3 is primarily a neuropeptide involved in stress and metabolic control, and INSL5 is widely distributed particularly in the gastrointestinal tract. These members of relaxin peptide family exert such effects binding to different kinds of receptors, classified as relaxin family peptide (RXFP) receptors: RXFP1, RXFP2, RXFP3, and RXFP4. These G protein-coupled receptors predominantly bind relaxin, INSL3, relaxin-3, and INSL-5, respectively. RXFP1 activates a wide spectrum of signaling pathways to generate second messengers that include cAMP and nitric oxide, whereas RXFP2 activates a subset of these pathways. Both RXFP3 and RXFP4 inhibit cAMP production, and RXFP3 activate MAP kinases. |
| map04927 | Cortisol synthesis and secretion | Cortisol is the main endogenous glucocorticoid, which affects a plethora of physiological functions, e.g., lipid and glucose metabolism, metabolic homeostasis and adaptation to stress. Cortisol production is primarily regulated by corticotropin (ACTH) in zona fasciculata. The stimulatory effect of ACTH on cortisol synthesis depends on cAMP dependent signaling, but also involves membrane depolarization and increased cytosolic Ca2+. Each of cAMP and Ca2+ induces the expression of StAR, stimulating intramitochondrial cholesterol transfer, as well as the steroidogenic enzymes in the pathway from cholesterol to cortisol (e.g., CHE, CYP17A1, CYP11B1). |
| map04928 | Parathyroid hormone synthesis, secretion and action | Parathyroid hormone (PTH) is a key regulator of calcium and phosphorus homeostasis. The principal regulators of PTH secretion are extracellular ionized calcium (Ca2+) and 1,25-dihydroxyvitamin D (1,25(OH)2D3). Under conditions of dietary Ca restriction, a decrement in serum Ca concentration induces release of PTH from the parathyroid gland. PTH acts on bone and kidney to stimulate bone turnover, increase the circulating levels of 1,25(OH)2D3 and calcium and inhibit the reabsorption of phosphate from the glomerular filtrate. This hormone exerts its actions via binding to the PTH/PTH-related peptide receptor (PTH1R). PTH1R primarily activates two sub-types of heterotrimeric Gproteins: Gs and Gq , which in turn regulate the activity of adenylyl cyclases and phospholipase C (PLC) that control the flow of cAMP/PKA and IP/PKC signaling cascades, respectively. |
| map04933 | AGE-RAGE signaling pathway in diabetic complications | Advanced glycation end products (AGEs) are a complex group of compounds produced through the non-enzymatic glycation and oxidation of proteins, lipids and nucleic acids, primarily due to aging and under certain pathologic condition such as huperglycemia. Some of the best chemically characterized AGEs include N-epsilon-carboxy-methyl-lysine (CML), N-epsilon-carboxy-ethyl-lysine (CEL), and Imidazolone. The major receptor for AGEs, known as receptor for advanced glycation end products (RAGE or AGER), belongs to the immunoglobulin superfamily and has been described as a pattern recognition receptor. AGE/RAGE signaling elicits activation of multiple intracellular signal pathways involving NADPH oxidase, protein kinase C, and MAPKs, then resulting in NF-kappaB activity. NF-kappa B promotes the expression of pro-inflammatory cytokines such as IL-1, IL-6 and TNF-alpha and a variety of atherosclerosis-related genes, including VCAM-1, tissue factor, VEGF, and RAGE. In addition, JAK-STAT-mediated and PI3K-Akt-dependent pathways are induced via RAGE, which in turn participate in cell proliferation and apoptosis respectively. Hypoxia-mediated induction of Egr-1 was also shown to require the AGE-RAGE interaction. The results of these signal transductions have been reported to be the possible mechanism that initates diabetic complications. |
| map04934 | Cushing syndrome | Cushing syndrome (CS) is a rare disorder resulting from prolonged exposure to excess glucocorticoids via exogenous and endogenous sources. The typical clinical features of CS are related to hypercortisolism and include accumulation of central fat, moon facies, neuromuscular weakness, osteoporosis or bone fractures, metabolic complications, and mood changes. Traditionally, endogenous CS is classified as adrenocorticotropic hormone (ACTH)-dependent (about 80%) or ACTH- independent (about 20%). Among ACTH-dependent forms, pituitary corticotroph adenoma (Cushing's disease) is most common. Most pituitary tumors are sporadic, resulting from monoclonal expansion of a single mutated cell. Recently recurrent activating somatic driver mutations in the ubiquitin-specific protease 8 gene (USP8) were identified in almost half of corticotroph adenoma. Germline mutations in MEN1 (encoding menin), AIP (encoding aryl-hydrocarbon receptor-interacting protein), PRKAR1A (encoding cAMP-dependent protein kinase type I alpha regulatory subunit) and CDKN1B (encoding cyclin-dependent kinase inhibitor 1B; also known as p27 Kip1) have been identified in familial forms of pituitary adenomas. However, the frequency of familial pituitary adenomas is less than 5% in patients with pituitary adenomas. Among ACTH-independent CS, adrenal adenoma is most common. Rare adrenal causes of CS include primary bilateral macronodular adrenal hyperplasia (BMAH) or primary pigmented nodular adrenocortical disease (PPNAD). |
| map04961 | Endocrine and other factor-regulated calcium reabsorption | Calcium (Ca2+) is essential for numerous physiological functions including intracellular signalling processes, neuronal excitability, muscle contraction and bone formation. Therefore, its homeostasis is finely maintained through the coordination of intestinal absorption, renal reabsorption, and bone resorption. In kidney, the late part of the distal convoluted tubule (DCT) and the connecting tubule (CNT) are the site of active Ca2+ transport and precisely regulate Ca2+ reabsorption. Following Ca2+ entry through TRPV5, Ca2+ bound to calbindin-D28K diffuses to the basolateral side, where it is extruded into the blood compartment through NCX1 and to a lesser extent PMCA1b. In the urinary compartment, both klotho and tissue kallikrein (TK) increase the apical abundance of TRPV5. In the blood compartment, PTH, 1,25(OH)2D3 and estrogen increase the transcription and protein expression of the luminal Ca2+ channels, calbindins, and the extrusion systems. |
| map04970 | Salivary secretion | Saliva has manifold functions in maintaining the integrity of the oral tissues, in protecting teeth from caries, in the tasting and ingestion of food, in speech and in the tolerance of tenures, for example. Salivary secretion occurs in response to stimulation by neurotransmitters released from autonomic nerve endings. There are two secretory pathways: protein exocytosis and fluid secretion. Sympathetic stimulation leads to the activation of adenylate cyclase and accumulation of intracellular cAMP. The elevation of cAMP causes the secretion of proteins such as amylase and mucin. In contrast, parasympathetic stimulation activates phospholipase C and causes the elevation of intracellular Ca2+, which leads to fluid secretion; that is, water and ion transport. Ca2+ also induces amylase secretion, but the amount is smaller than that induced by cAMP. |
| map04971 | Gastric acid secretion | Gastric acid is a key factor in normal upper gastrointestinal functions, including protein digestion and calcium and iron absorption, as well as providing some protection against bacterial infections. The principal stimulants of acid secretion at the level of the parietal cell are histamine (paracrine), gastrin (hormonal), and acetycholine (ACh; neurocrine). Stimulation of acid secretion typically involves an initial elevation of intracellular calcium and cAMP, followed by activation of protein kinase cascades, which trigger the translocation of the proton pump, H+,K+-ATPase, from cytoplasmic tubulovesicles to the apical plasma membrane and thereby H+ secretion into the stomach lumen. |
| map04972 | Pancreatic secretion | The pancreas performs both exocrine and endocrine functions. The exocrine pancreas consists of two parts, the acinar and duct cells. The primary functions of pancreatic acinar cells are to synthesize and secrete digestive enzymes. Stimulation of the cell by secretagogues such as acetylcholine (ACh) and cholecystokinin (CCK) causes the generation of an intracellular Ca2+ signal. This signal, in turn, triggers the fusion of the zymogen granules with the apical plasma membrane, leading to the polarised secretion of the enzymes. The major task of pancreatic duct cells is the secretion of fluid and bicarbonate ions (HCO3-), which neutralize the acidity of gastric contents that enter the duodenum. An increase in intracellular cAMP by secretin is one of the major signals of pancreatic HCO3- secretion. Activation of the CFTR Cl- channel and the CFTR-dependent Cl-/HCO3- exchange activities is responsible for cAMP-induced HCO3- secretion. |
| map05010 | Alzheimer disease | Alzheimer disease (AD) is a chronic disorder that slowly destroys neurons and causes serious cognitive disability. AD is associated with senile plaques and neurofibrillary tangles (NFTs). Amyloid-beta (Abeta), a major component of senile plaques, has various pathological effects on cell and organelle function. The extracellular Abeta oligomers may activate caspases through activation of cell surface death receptors. Alternatively, intracellular Abeta may contribute to pathology by facilitating tau hyper-phosphorylation, disrupting mitochondria function, and triggering calcium dysfunction. To date genetic studies have revealed four genes that may be linked to autosomal dominant or familial early onset AD (FAD). These four genes include: amyloid precursor protein (APP), presenilin 1 (PS1), presenilin 2 (PS2) and apolipoprotein E (ApoE). All mutations associated with APP and PS proteins can lead to an increase in the production of Abeta peptides, specfically the more amyloidogenic form, Abeta42. FAD-linked PS1 mutation downregulates the unfolded protein response and leads to vulnerability to ER stress. |
| map05016 | Huntington disease | Huntington disease (HD) is an autosomal-dominant neurodegenerative disorder that primarily affects medium spiny striatal neurons (MSN). The symptoms are choreiform, involuntary movements, personality changes and dementia. HD is caused by a CAG repeat expansion in the IT15gene, which results in a long stretch of polyglutamine close to the amino-terminus of the HD protein huntingtin (Htt). Mutant Htt (mHtt) has effects both in the cytoplasm and in the nucleus. In the cytoplasm, full-length mHtt can interfere with BDNF vesicular transport on microtubules. This mutant protein also may lead to abnormal endocytosis and secretion in neurons, because normal Htt form a complex with the proteins Hip1, clathrin and AP2 that are involved in endocytosis. In addition, mHtt affects Ca2+ signaling by sensitizing InsP3R1 to activation by InsP3, stimulating NMDAR activity, and destabilizing mitochondrial Ca2+ handling. The mHtt translocates to the nucleus, where it forms intranuclear inclusions. Nuclear toxicity is believed to be caused by interference with gene transcription, leading to loss of transcription of neuroprotective molecules such as BDNF. While mHtt binds to p53 and upregulates levels of nuclear p53 as well as p53 transcriptional activity. Augmented p53 mediates mitochondrial dysfunction. |
| map05110 | Vibrio cholerae infection | Cholera toxin (CTX) is one of the main virulence factors of Vibrio cholerae. Once secreted, CTX B-chain (CTXB) binds to ganglioside GM1 on the surface of the host's cells. After binding takes place, the entire CTX complex is carried from plasma membrane (PM) to endoplasmic reticulum (ER). In the ER, the A-chain (CTXA) is recognized by protein disulfide isomerase (PDI), unfolded, and delivered to the membrane where the membrane-associated ER-oxidase, Ero1, oxidizes PDI to release the CTXA into the protein-conducting channel, Sec61. CTXA is then retro-translocated to the cytosol and induces water and electrolyte secretion by increasing cAMP levels via adenylate cyclase (AC) to exert toxicity.Other than CTX, Vibrio cholerae generates several toxins that are perilous to eukaryotic cells. Zonula occludens toxin (ZOT) causes tight junction disruption through protein kinase C-dependent actin polymerization. RTX toxin (RtxA) causes actin depolymerization by covalently cross-linking actin monomers into dimers, trimers, and higher multimers. Vibrio cholerae cytolysin (VCC) is an important pore-forming toxin. The assembly of VCC anion channels in cells cause vacuolization and lysis. |
| map05142 | Chagas disease (American trypanosomiasis) | Trypanosoma cruzi is an intracellular protozoan parasite that causes Chagas disease. The parasite life cycle involves hematophagous reduviid bugs as vectors. Once parasites enter the host body, they invade diverse host cells including cardiomyocytes. Establishment of infection depends on various parasite molecules such as cruzipain, oligopeptidase B, and trans-sialidase that activate Ca2+ signaling. Internalized parasites escape from the parasitophorous vacuole using secreted pore-forming TcTOX molecule and replicate in the cytosol. Multiplied parasites eventually lyse infected host cells and are released in the circulation. During these events, the parasites manipulate host innate immunity and elicit cardiomyocyte hypertrophy. T lymphocyte responses are also disturbed. |
| map05143 | African trypanosomiasis | Trypanosoma brucei, the parasite responsible for African trypanosomiasis (sleeping sickness), are spread by the tsetse fly in sub-Saharan Africa. The parasites are able to pass through the blood-brain barrier and cause neurological damage by inducing cytokines like TNF alpha, IFN gamma, and IL1. These cytokines and other metabolites such as nitric oxide and somnogenic prostaglandin D2 disturb circadian rhythms in patients with African trypanosomiasis. |
| map05146 | Amoebiasis | Entamoeba histolytica, an extracellular protozoan parasite is a human pathogen that invades the intestinal epithelium. Infection occurs on ingestion of contaminated water and food. The pathogenesis of amoebiasis begins with parasite attachment and disruption of the intestinal mucus layer, followed by apoptosis of host epithelial cells. Intestinal tissue destruction causes severe dysentery and ulcerations in amoebic colitis. Several amoebic proteins such as lectins, cysteine proteineases, and amoebapores are associated with the invasion process. The parasite can cause extraintestinal infection like amoebic liver abscess by evading immune response. |
| map05163 | Human cytomegalovirus infection | Human cytomegalovirus (HCMV) is an enveloped, double-stranded DNA virus that is a member of beta-herpesvirus family. HCMV is best known for causing significant morbidity and mortality in immunocompromised populations. As with other herpesviruses, HCMV gB and gH/gL envelope glycoproteins are essential for virus entry. HCMV gB could activate the PDGFRA, and induce activation of the oncogenic PI3-K/AKT pathway. Though it is unlikely that HCMV by itself can act as an oncogenic factor, HCMV may have an oncomodulatory role, to catalyze an oncogenic process that has already been initiated. US28, one of the four HCMV-encoded vGPCRs (US27, US28, UL33 and UL78), also has a specific role in the oncomodulatory properties. In addition, HCMV has developed numerous mechanisms for manipulating the host immune system. The virally encoded US2, US3, US6 and US11 gene products all interfere with major histocompatibility complex (MHC) class I antigen presentation. HCMV encodes several immediate early (IE) antiapoptotic proteins (IE1, IE2, vMIA and vICA). These proteins might avoid immune clearance of infected tumor cells by cytotoxic lymphocytes and NK cells. |
| map05167 | Kaposi sarcoma-associated herpesvirus infection | Kaposi sarcoma-associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV-8), is the most recently identified human tumor virus, and is associated with the pathogenesis of Kaposi's sarcoma (KS), primary effusion lymphoma (PEL), and Multicentric Castleman's disease (MCD). Like all other herpesviruses, KSHV displays two modes of life cycle, latency and lytic replication, which are characterized by the patterns of viral gene expression. Genes expressed in latency (LANA, v-cyclin, v-FLIP, Kaposins A, B and C and viral miRNAs) are mainly thought to facilitate the establishment of life long latency in its host and survival against the host innate, and adaptive immune surveillance mechanisms. Among the viral proteins shown to be expressed during lytic replication are potent signaling molecules such as vGPCR, vIL6, vIRFs, vCCLs, K1 and K15, which have been implicated experimentally in the angiogenic and inflammatory phenotype observed in KS lesions. Several of these latent viral and lytic proteins are known to transform host cells, linking KSHV with the development of severe human malignancies. |
| map05169 | Epstein-Barr virus infection | Epstein-Barr virus (EBV) is a gamma-herpes virus that widely infects human populations predominantly at an early age but remains mostly asymptomatic. EBV has been linked to a wide spectrum of human malignancies, including nasopharyngeal carcinoma and other hematologic cancers, like Hodgkin's lymphoma, Burkitt's lymphoma (BL), B-cell immunoblastic lymphoma in HIV patients, and posttransplant-associated lymphoproliferative diseases. EBV has the unique ability to establish life-long latent infection in primary human B lymphocytes. During latent infection, EBV expresses a small subset of genes, including 6 nuclear antigens (EBNA-1, -2, -3A, -3B, -3C, and -LP), 3 latent membrane proteins (LMP-1, -2A, and -2B), 2 small noncoding RNAs (EBER-1 and 2). On the basis of these latent gene expression, three different latency patterns associated with the types of cancers are recognized. |
| map05170 | Human immunodeficiency virus 1 infection | Human immunodeficiency virus type 1 (HIV-1) , the causative agent of AIDS (acquired immunodeficiency syndrome), is a lentivirus belonging to the Retroviridae family. The primary cell surface receptor for HIV-1, the CD4 protein, and the co-receptor for HIV-1, either CCR5 or CXCR4, are found on macrophages and T lymphocytes. At the earliest step, sequential binding of virus envelope (Env) glycoprotein gp120 to CD4 and the co-receptor CCR5 or CXCR4 facilitates HIV-1 entry and has the potential to trigger critical signaling that may favor viral replication. At advanced stages of the disease, HIV-1 infection results in dramatic induction of T-cell (CD4+ T and CD8+ T cell) apoptosis both in infected and uninfected bystander T cells, a hallmark of HIV-1 pathogenesis. On the contrary, macrophages are resistant to the cytopathic effect of HIV-1 and produce virus for longer periods of time. |
| map05200 | Pathways in cancer | NA |
| map05214 | Glioma | Gliomas are the most common of the primary brain tumors and account for more than 40% of all central nervous system neoplasms. Gliomas include tumours that are composed predominantly of astrocytes (astrocytomas), oligodendrocytes (oligodendrogliomas), mixtures of various glial cells (for example,oligoastrocytomas) and ependymal cells (ependymomas). The most malignant form of infiltrating astrocytoma - glioblastoma multiforme (GBM) - is one of the most aggressive human cancers. GBM may develop de novo (primary glioblastoma) or by progression from low-grade or anaplastic astrocytoma (secondary glioblastoma). Primary glioblastomas develop in older patients and typically show genetic alterations (EGFR amplification, p16/INK4a deletion, and PTEN mutations) at frequencies of 24-34%. Secondary glioblastomas develop in younger patients and frequently show overexpression of PDGF and CDK4 as well as p53 mutations (65%) and loss of Rb playing major roles in such transformations. Loss of PTEN has been implicated in both pathways, although it is much more common in the pathogenesis of primary GBM. |
| map05223 | Non-small cell lung cancer | Lung cancer is a leading cause of cancer death among men and women in industrialized countries. Non-small-cell lung cancer (NSCLC) accounts for approximately 85% of lung cancer and represents a heterogeneous group of cancers, consisting mainly of squamous cell (SCC), adeno (AC) and large-cell carcinoma. Molecular mechanisms altered in NSCLC include activation of oncogenes, such as K-RAS, EGFR and EML4-ALK, and inactivation of tumorsuppressor genes, such as p53, p16INK4a, RAR-beta, and RASSF1. Point mutations within the K-RAS gene inactivate GTPase activity and the p21-RAS protein continuously transmits growth signals to the nucleus. Mutations or overexpression of EGFR leads to a proliferative advantage. EML4-ALK fusion leads to constitutive ALK activation, which causes cell proliferation, invasion, and inhibition of apoptosis. Inactivating mutation of p53 can lead to more rapid proliferation and reduced apoptosis. The protein encoded by the p16INK4a inhibits formation of CDK-cyclin-D complexes by competitive binding of CDK4 and CDK6. Loss of p16INK4a expression is a common feature of NSCLC. RAR-beta is a nuclear receptor that bears vitamin-A-dependent transcriptional activity. RASSF1A is able to form heterodimers with Nore-1, an RAS effector.Therefore loss of RASSF1A might shift the balance of RAS activity towards a growth-promoting effect. |
| map05225 | Hepatocellular carcinoma | Hepatocellular carcinoma (HCC) is a major type of primary liver cancer and one of the rare human neoplasms etiologically linked to viral factors. It has been shown that, after HBV/HCV infection and alcohol or aflatoxin B1 exposure, genetic and epigenetic changes occur. The recurrent mutated genes were found to be highly enriched in multiple key driver signaling processes, including telomere maintenance, TP53, cell cycle regulation, the Wnt/beta-catenin pathway (CTNNB1 and AXIN1), the phosphatidylinositol-3 kinase (PI3K)/AKT/mammalian target of rapamycin (mTOR) pathway. Recent studies using whole-exome sequencing have revealed recurrent mutations in new driver genes involved in the chromatin remodelling (ARID1A and ARID2) and the oxidative stress (NFE2L2) pathways. |

