
- DEPOD Home
- About DEPOD
- User Manual
- Human Phosphatases
- Protein Substrates
- Non-Protein Substrates
- Pathway Mapping
- Dephosphorylation Network
- ID Mapping
- Download
- Acknowledgement
- Contact
- Imprint
- Links
- Ensembl
- Entrez Gene
- HGNC
- QuickGO
- Gene Expression Atlas
- OMIM
- UniProt
- IntEnz
- BRENDA
- LocDB
- M-CSA
- InterPro
- Pfam
- CATH
- Gene3D
- PDB
- PDBe
- DrugPort
- ModBase
- SwissModel
- ChEMBL
- Phospho.ELM
- PhosphoSitePlus
- NetworKIN
- HPRD
- PSICQUIC
- STRING
- IntAct
- MINT
- KEGG Pathway
- Reactome Pathway
- Pathway Commons
- PubMed
- ArchSchema
- Cytoscape Web
- Europe PMC
- EKPD
- SwissLipids
 Statistics
Statistics
194 phosphatases have substrate data;
--------------------------------
336 protein substrates;
83 non-protein substrates;
1215 dephosphorylation interactions;
--------------------------------
299 KEGG pathways;
876 Reactome pathways;
--------------------------------
last scientific update:
11 Mar, 2019

Top
FBP1
| Gene Name | FBP1 (QuickGO) | (A quick tutorial to explore the interctive visulaization) |
| Synonyms | FBP1, FBP | |
| Protein Name | FBP1 |
|
| Alternative Name(s) |
Fructose-1,6-bisphosphatase 1;FBPase 1;3.1.3.11;D-fructose-1,6-bisphosphate 1-phosphohydrolase 1;Liver FBPase; | |
| EntrezGene ID | 2203 (Comparitive Toxicogenomics) | |
| UniProt AC (Human) | P09467 (protein sequence) | |
| Enzyme Class | EC 3.1.3.11 (BRENDA | |
| Molecular Weight | 36842 Dalton | |
| Protein Length | 338 amino acids (AA) | |
| Genome Browsers | NCBI | ENSG00000165140 (Ensembl) | UCSC | |
| Crosslinking annotations | Query our ID-mapping table | |
| Orthologues | Quest For Orthologues (QFO) | GeneTree | |
| Classification | Superfamily: adenosine_fructose_inositol-x-phosphatases (AFIxP) | Historic class: FBPase class 1 | CATH ID: 3.30.540.10, 3.40.190.80 | SCOP Fold: CP | |
| Phosphatase activity | active | Catalytic signature motif: unknown | |
| Phosphorylation Network | Visualize | |
| Domain organization, Expression, Diseases | (show / hide) |
| Protein Domain | InterPro | Pfam | SMART | 3D Structure | PDB | DrugPort | ModBase | SwissModel |
| Polypeptide organization (Pfam) | No Pfam domain found | ||
| Catalytic Site | Mechanism and Catalytic Site Atlas | ||
| Gene Expression | Gene Expression Atlas | Diseases | OMIM | DisGeNet | ClinVar |
| Protein-protein interactions | STRING | IntAct | MINT | BioGRID | ||
| Phosphosites | Phospho.ELM | PhosphoSitePlus | ||
| Localization, Function, Catalytic activity and Sequence | (show / hide) |
| Localization (UniProt annotation) | |||
| N/A | |||
| Function (UniProt annotation) | |||
| Catalyzes the hydrolysis of fructose 1,6-bisphosphate tofructose 6-phosphate in the presence of divalent cations, actingas a rate-limiting enzyme in gluconeogenesis Plays a role inregulating glucose sensing and insulin secretion of pancreaticbeta-cells Appears to modulate glycerol gluconeogenesis in liverImportant regulator of appetite and adiposity; increasedexpression of the protein in liver after nutrient excess increasescirculating satiety hormones and reduces appetite-stimulatingneuropeptides and thus seems to provide a feedback mechanism tolimit weight gain | |||
| Catalytic Activity (UniProt annotation) | |||
| D-fructose 1,6-bisphosphate + H(2)O = D-fructose 6-phosphate + phosphate | |||
| Protein Sequence | |||
| MADQAPFDTDVNTLTRFVMEEGRKARGTGELTQLLNSLCTAVKAISSAVRKAGIAHLYGIAGSTNVTGDQVKKLDVLSND LVMNMLKSSFATCVLVSEEDKHAIIVEPEKRGKYVVCFDPLDGSSNIDCLVSVGTIFGIYRKKSTDEPSEKDALQPGRNL VAAGYALYGSATMLVLAMDCGVNCFMLDPAIGEFILVDKDVKIKKKGKIYSLNEGYARDFDPAVTEYIQRKKFPPDNSAP YGARYVGSMVADVHRTLVYGGIFLYPANKKSPNGKLRLLYECNPMAYVMEKAGGMATTGKEAVLDVIPTDIHQRAPVILG SPDDVLEFLKVYEKHSAQ |
| Motif information from Eukaryotic Linear Motif atlas (ELM) | (show / hide) |
| Gene Ontology (P: Process; F: Function and C: Component terms) | (show / hide) | |
| GO:0000122 | P:negative regulation of transcription by RNA polymerase II |
| GO:0001191 | F:transcriptional repressor activity |
| GO:0005634 | RNA polymerase II transcription factor binding |
| GO:0005737 | C:nucleus |
| GO:0005829 | C:cytoplasm |
| GO:0005986 | C:cytosol |
| GO:0006000 | P:sucrose biosynthetic process |
| GO:0006002 | P:fructose metabolic process |
| GO:0006094 | P:fructose 6-phosphate metabolic process |
| GO:0006111 | P:gluconeogenesis |
| GO:0016208 | P:regulation of gluconeogenesis |
| GO:0016311 | F:AMP binding |
| GO:0030308 | P:dephosphorylation |
| GO:0030388 | P:negative regulation of cell growth |
| GO:0035690 | P:fructose 1,6-bisphosphate metabolic process |
| GO:0042132 | P:cellular response to drug |
| GO:0042802 | F:fructose 1,6-bisphosphate 1-phosphatase activity |
| GO:0045820 | F:identical protein binding |
| GO:0046580 | P:negative regulation of glycolytic process |
| GO:0046872 | P:negative regulation of Ras protein signal transduction |
| GO:0048029 | F:metal ion binding |
| GO:0051289 | F:monosaccharide binding |
| GO:0070062 | P:protein homotetramerization |
| GO:0071286 | C:extracellular exosome |
[Feedback|Contribution]
| Substrate ChEBI-ID | Substrate KEGG ID | DephosphoSite | Bioassay Type | PubMed ID | View in Europe PMC | Reliability of the interaction | Molecule |
| CHEBI:16905 | C00354 | C-1 position | In vitro | 8387495, 10222032, | Europe PMC |   | 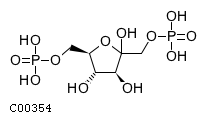 |
| CHEBI:37736 | C00354 | C-1 position | In vitro | 8387495, 10222032, | Europe PMC |   |  |
| Pathway ID | Pathway Name | Pathway Description (KEGG) |
| hsa00010 | Glycolysis / Gluconeogenesis | Glycolysis is the process of converting glucose into pyruvate and generating small amounts of ATP (energy) and NADH (reducing power). It is a central pathway that produces important precursor metabolites: six-carbon compounds of glucose-6P and fructose-6P and three-carbon compounds of glycerone-P, glyceraldehyde-3P, glycerate-3P, phosphoenolpyruvate, and pyruvate [MD:M00001]. Acetyl-CoA, another important precursor metabolite, is produced by oxidative decarboxylation of pyruvate [MD:M00307]. When the enzyme genes of this pathway are examined in completely sequenced genomes, the reaction steps of three-carbon compounds from glycerone-P to pyruvate form a conserved core module [MD:M00002], which is found in almost all organisms and which sometimes contains operon structures in bacterial genomes. Gluconeogenesis is a synthesis pathway of glucose from noncarbohydrate precursors. It is essentially a reversal of glycolysis with minor variations of alternative paths [MD:M00003]. |
| hsa00030 | Pentose phosphate pathway | The pentose phosphate pathway is a process of glucose turnover that produces NADPH as reducing equivalents and pentoses as essential parts of nucleotides. There are two different phases in the pathway. One is irreversible oxidative phase in which glucose-6P is converted to ribulose-5P by oxidative decarboxylation, and NADPH is generated [MD:M00006]. The other is reversible non-oxidative phase in which phosphorylated sugars are interconverted to generate xylulose-5P, ribulose-5P, and ribose-5P [MD:M00007]. Phosphoribosyl pyrophosphate (PRPP) formed from ribose-5P [MD:M00005] is an activated compound used in the biosynthesis of histidine and purine/pyrimidine nucleotides. This pathway map also shows the Entner-Doudoroff pathway where 6-P-gluconate is dehydrated and then cleaved into pyruvate and glyceraldehyde-3P [MD:M00008]. |
| hsa00051 | Fructose and mannose metabolism | |
| hsa01100 | Metabolic pathways | |
| hsa01200 | Carbon metabolism | Carbon metabolism is the most basic aspect of life. This map presents an overall view of central carbon metabolism, where the number of carbons is shown for each compound denoted by a circle, excluding a cofactor (CoA, CoM, THF, or THMPT) that is replaced by an asterisk. The map contains carbon utilization pathways of glycolysis (map00010), pentose phosphate pathway (map00030), and citrate cycle (map00020), and six known carbon fixation pathways (map00710 and map00720) as well as some pathways of methane metabolism (map00680). The six carbon fixation pathways are: (1) reductive pentose phosphate cycle (Calvin cycle) in plants and cyanobacteria that perform oxygenic photosynthesis, (2) reductive citrate cycle in photosynthetic green sulfur bacteria and some chemolithoautotrophs, (3) 3-hydroxypropionate bi-cycle in photosynthetic green nonsulfur bacteria, two variants of 4-hydroxybutyrate pathways in Crenarchaeota called (4) hydroxypropionate-hydroxybutyrate cycle and (5) dicarboxylate-hydroxybutyrate cycle, and (6) reductive acetyl-CoA pathway in methanogenic bacteria. |
| hsa04152 | AMPK signaling pathway | AMP-activated protein kinase (AMPK) is a serine threonine kinase that is highly conserved through evolution. AMPK system acts as a sensor of cellular energy status. It is activated by increases in the cellular AMP:ATP ratio caused by metabolic stresses that either interfere with ATP production (eg, deprivation for glucose or oxygen) or that accelerate ATP consumption (eg, muscle contraction). Several upstream kinases, including liver kinase B1 (LKB1), calcium/calmodulin kinase kinase-beta (CaMKK beta), and TGF-beta-activated kinase-1 (TAK-1), can activate AMPK by phosphorylating a threonine residue on its catalytic alpha-subunit. Once activated, AMPK leads to a concomitant inhibition of energy-consuming biosynthetic pathways, such as protein, fatty acid and glycogen synthesis, and activation of ATP-producing catabolic pathways, such as fatty acid oxidation and glycolysis. |
| hsa04910 | Insulin signaling pathway | Insulin binding to its receptor results in the tyrosine phosphorylation of insulin receptor substrates (IRS) by the insulin receptor tyrosine kinase (INSR). This allows association of IRSs with the regulatory subunit of phosphoinositide 3-kinase (PI3K). PI3K activates 3-phosphoinositide-dependent protein kinase 1 (PDK1), which activates Akt, a serine kinase. Akt in turn deactivates glycogen synthase kinase 3 (GSK-3), leading to activation of glycogen synthase (GYS) and thus glycogen synthesis. Activation of Akt also results in the translocation of GLUT4 vesicles from their intracellular pool to the plasma membrane, where they allow uptake of glucose into the cell. Akt also leads to mTOR-mediated activation of protein synthesis by eIF4 and p70S6K. The translocation of GLUT4 protein is also elicited through the CAP/Cbl/TC10 pathway, once Cbl is phosphorylated by INSR.Other signal transduction proteins interact with IRS including GRB2. GRB2 is part of the cascade including SOS, RAS, RAF and MEK that leads to activation of mitogen-activated protein kinase (MAPK) and mitogenic responses in the form of gene transcription. SHC is another substrate of INSR. When tyrosine phosphorylated, SHC associates with GRB2 and can thus activate the RAS/MAPK pathway independently of IRS-1. |
| hsa04922 | Glucagon signaling pathway | Glucagon is conventionally regarded as a counterregulatory hormone for insulin and plays a critical anti-hypoglycemic role by maintaining glucose homeostasis in both animals and humans. To increase blood glucose, glucagon promotes hepatic glucose output by increasing glycogenolysis and gluconeogenesis and by decreasing glycogenesis and glycolysis in a concerted fashion via multiple mechanisms. Glucagon also stimulates hepatic mitochondrial beta-oxidation to supply energy for glucose production. Glucagon performs its main effect via activation of adenylate cyclase. The adenylate-cyclase-derived cAMP activates protein kinase A (PKA), which then phosphorylates downstream targets, such as cAMP response element binding protein (CREB) and the bifunctional enzyme 6-phosphofructo-2-kinase/ fructose-2,6-bisphosphatase (one of the isoforms being PFK/FBPase 1, encoded by PFKFB1). |
| Pathway ID | Pathway Name | Pathway Description (KEGG) |
| R-HSA-70263 | Gluconeogenesis. | The reactions of gluconeogenesis convert mitochondrial pyruvate to cytosolic glucose 6-phosphate which in turn can be hydrolyzed to glucose and exported from the cell. Gluconeogenesis is confined to cells of the liver and kidney and enables glucose synthesis from molecules such as lactate and alanine and other amino acids when exogenous glucose is not available (reviewed, e.g., by Gerich 1993). The process of gluconeogenesis as diagrammed below occurs in two parts: a network of reactions converts mitochondrial pyruvate to cytosolic phosphoenolpyruvate; then phosphoenolpyruvate is converted to glucose 6-phosphate in a single sequence of cytosolic reactions. Three variants of the first part of the process are physiologically important. 1) A series of transport and transamination reactions convert mitochondrial oxaloacetate to cytosolic oxaloacetate which is converted to phosphoenolpyruvate by a hormonally regulated, cytosolic isoform of phosphoenolpyruvate carboxykinase. This variant allows regulated glucose synthesis from lactate. 2) Mitochondrial oxaloacetate is reduced to malate, which is exported to the cytosol and re-oxidized to oxaloacetate. This variant provides reducing equivalents to the cytosol, needed for glucose synthesis from amino acids such as alanine and glutamine. 3) Constitutively expressed mitochondrial phosphoenolpyruvate carboxykinase catalyzes the conversion of mitochondrial oxaloacetate to phosphoenolpyruvate which is then transported to the cytosol. The exact path followed by any one molecule of pyruvate through this reaction network is determined by the tissue in which the reactions are occurring, the source of the pyruvate, and the physiological stress that triggered gluconeogenesis. In all cases, the synthesis of glucose from two molecules of pyruvate requires the generation and consumption of two reducing equivalents as cytosolic NADH + H+. For pyruvate derived from lactate (variants 1 and 3), NADH + H+ is generated with the oxidation of lactate to pyruvate in the cytosol (a reaction of pyruvate metabolism not shown in the diagram). For pyruvate derived from amino acids (variant 2), mitochondrial NADH + H+ generated by glutamate dehydrogenase (a reaction of amino acid metabolism, not shown) is used to reduce oxaloacetate to malate, which is transported to the cytosol and re-oxidized, generating cytosolic NADH + H+. The synthesis of glucose from pyruvate also requires the consumption of six high-energy phosphates, four from ATP and two from GTP. In the second part of gluconeogenesis, cytosolic phosphoenolpyruvate, however derived, is converted to fructose 1,6-bisphosphate by reactions that are the reverse of steps of glycolysis. Hydrolysis of fructose 1,6-bisphosphate to fructose 6-phosphate is catalyzed by fructose 1,6-bisphosphatase, and fructose 6-phosphate is reversibly isomerized to glucose 6-phosphate |
| Interacting partner | Detection method | Interaction type | PubMed IDs | Interaction Score | Source |
| ASL | Two-hybrid, two hybrid | physical, physical association | 21988832, (Europe PMC) | 0.37 | BioGRID, IntAct |
| ATP5MF | Two-hybrid | physical | 16169070, (Europe PMC) | NA | BioGRID |
| BCL2L1 | Two-hybrid, two hybrid | physical, physical association | 21900206, (Europe PMC) | 0.37 | BioGRID, IntAct, MINT |
| BIN1 | Protein-peptide, Reconstituted Complex, Two-hybrid, filter binding, peptide array, pull down, two hybrid | physical, physical association | 16275660, (Europe PMC) | 0.63 | BioGRID, IntAct |
| CCDC68 | Affinity Capture-MS | physical | 26186194, 28514442, (Europe PMC) | NA | BioGRID |
| CROT | Affinity Capture-MS | physical | 28514442, (Europe PMC) | NA | BioGRID |
| CSNK1E | Two-hybrid, two hybrid | physical, physical association | 21900206, (Europe PMC) | 0.37 | BioGRID, IntAct, MINT |
| DYNC1I1 | Two-hybrid, two hybrid | physical, physical association | 21900206, (Europe PMC) | 0.37 | BioGRID, IntAct, MINT |
| FBP1 | Co-crystal Structure, Two-hybrid, split luciferase complementation, two hybrid, two hybrid array, two hybrid bait and prey pooling approach, two hybrid pooling approach, two hybrid prey pooling approach, validated two hybrid | physical, physical association | 16169070, 16189514, 21516116, 2164670, 25416956, 25910212, , (Europe PMC) | 0.91 | BioGRID, IntAct, MINT |
| FBP2 | Affinity Capture-MS, Two-hybrid, split luciferase complementation, two hybrid array, two hybrid bait and prey pooling approach, two hybrid prey pooling approach, validated two hybrid | physical, physical association | 25416956, 25910212, 26186194, 28514442, , (Europe PMC) | 0.81 | BioGRID, IntAct |
| FXR2 | Two-hybrid, two hybrid pooling approach | physical, physical association | 16189514, (Europe PMC) | 0.37 | BioGRID, IntAct |
| GIMAP2 | Affinity Capture-MS | physical | 28514442, (Europe PMC) | NA | BioGRID |
| HDAC6 | Two-hybrid, two hybrid | physical, physical association | 21900206, (Europe PMC) | 0.37 | BioGRID, IntAct, MINT |
| HSPA8 | Two-hybrid, two hybrid pooling approach | physical, physical association | 16169070, (Europe PMC) | 0.37 | BioGRID, IntAct |
| KLRC2 | Two-hybrid, two hybrid array | physical, physical association | 21988832, (Europe PMC) | 0.37 | BioGRID, IntAct |
| LINC01667 | Affinity Capture-MS | physical | 26186194, 28514442, (Europe PMC) | NA | BioGRID |
| LNX1 | Two-hybrid, two hybrid pooling approach | physical, physical association | 16189514, (Europe PMC) | 0.37 | BioGRID, IntAct |
| PCNX4 | Two-hybrid | physical | 16189514, (Europe PMC) | NA | BioGRID |
| POT1 | Two-hybrid, bimolecular fluorescence complementation | physical, physical association | 21044950, (Europe PMC) | 0.37 | BioGRID, IntAct |
| PRKN | Biochemical Activity | physical | 21209200, (Europe PMC) | NA | BioGRID |
| PTK2 | Two-hybrid, two hybrid | physical, physical association | 21900206, (Europe PMC) | 0.37 | BioGRID, IntAct, MINT |
| RNF183 | Two-hybrid, two hybrid pooling approach | physical, physical association | 16189514, (Europe PMC) | 0.37 | BioGRID, IntAct |
| SAMD7 | Affinity Capture-MS | physical | 28514442, (Europe PMC) | NA | BioGRID |
| SLAMF8 | Affinity Capture-MS | physical | 28514442, (Europe PMC) | NA | BioGRID |
| ST6GALNAC5 | Affinity Capture-MS | physical | 26186194, 28514442, (Europe PMC) | NA | BioGRID |
| TERF1 | Two-hybrid, bimolecular fluorescence complementation | physical, physical association | 21044950, (Europe PMC) | 0.37 | BioGRID, IntAct |
| TIGD7 | Affinity Capture-MS | physical | 28514442, (Europe PMC) | NA | BioGRID |
| Interacting partner | Detection method | Interaction type | PubMed IDs | Interaction Score | Source |
| APC | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| ASCC2 | Two-hybrid, two hybrid pooling approach | physical, physical association | 16169070, (Europe PMC) | 0.37 | BioGRID, IntAct |
| ASL | Two-hybrid, two hybrid | physical, physical association | 21988832, (Europe PMC) | 0.37 | BioGRID, IntAct |
| ATP5J2 | two hybrid pooling approach | physical association | 16169070, (Europe PMC) | 0.37 | IntAct |
| AXIN2 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| BCL10 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| BCL2L1 | Two-hybrid, two hybrid | physical, physical association | 21900206, (Europe PMC) | 0.37 | BioGRID, IntAct, MINT |
| BIN1 | Protein-peptide, Reconstituted Complex, Two-hybrid, filter binding, peptide array, pull down, two hybrid | physical, physical association | 16275660, (Europe PMC) | 0.63 | BioGRID, IntAct |
| BMPR1A | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| BRAF | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| BUB1 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| CSNK1E | Two-hybrid, two hybrid | physical, physical association | 21900206, (Europe PMC) | 0.37 | BioGRID, IntAct, MINT |
| CTNNA1 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| DCC | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| DYNC1I1 | Two-hybrid, two hybrid | physical, physical association | 21900206, (Europe PMC) | 0.37 | BioGRID, IntAct, MINT |
| EPAS1 | anti bait coimmunoprecipitation, anti tag coimmunoprecipitation, pull down | direct interaction, physical association | 25043030, (Europe PMC) | 0.60 | IntAct |
| ERBB2 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| FBP1 | Co-crystal Structure, Two-hybrid, split luciferase complementation, two hybrid, two hybrid array, two hybrid bait and prey pooling approach, two hybrid pooling approach, two hybrid prey pooling approach, validated two hybrid | physical, physical association | 16169070, 16189514, 21516116, 2164670, 25416956, 25910212, , (Europe PMC) | 0.91 | BioGRID, IntAct, MINT |
| FBP2 | Affinity Capture-MS, Two-hybrid, split luciferase complementation, two hybrid array, two hybrid bait and prey pooling approach, two hybrid prey pooling approach, validated two hybrid | physical, physical association | 25416956, 25910212, 26186194, 28514442, , (Europe PMC) | 0.81 | BioGRID, IntAct |
| FBXW7 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| FLCN | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| FXR2 | Two-hybrid, two hybrid pooling approach | physical, physical association | 16189514, (Europe PMC) | 0.37 | BioGRID, IntAct |
| HDAC6 | Two-hybrid, two hybrid | physical, physical association | 21900206, (Europe PMC) | 0.37 | BioGRID, IntAct, MINT |
| HIF1A | anti bait coimmunoprecipitation, anti tag coimmunoprecipitation, pull down | direct interaction, physical association | 25043030, (Europe PMC) | 0.60 | IntAct |
| HSPA8 | Two-hybrid, two hybrid pooling approach | physical, physical association | 16169070, (Europe PMC) | 0.37 | BioGRID, IntAct |
| KLRC2 | Two-hybrid, two hybrid array | physical, physical association | 21988832, (Europe PMC) | 0.37 | BioGRID, IntAct |
| LNX1 | Two-hybrid, two hybrid pooling approach | physical, physical association | 16189514, (Europe PMC) | 0.37 | BioGRID, IntAct |
| MLH3 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| MSH2 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| PCNX4 | two hybrid pooling approach | physical association | 16189514, (Europe PMC) | 0.37 | IntAct |
| POT1 | Two-hybrid, bimolecular fluorescence complementation | physical, physical association | 21044950, (Europe PMC) | 0.37 | BioGRID, IntAct |
| PTK2 | Two-hybrid, two hybrid | physical, physical association | 21900206, (Europe PMC) | 0.37 | BioGRID, IntAct, MINT |
| PTPRJ | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| RB1 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| RNF183 | Two-hybrid, two hybrid pooling approach | physical, physical association | 16189514, (Europe PMC) | 0.37 | BioGRID, IntAct |
| STK11 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| TERF1 | Two-hybrid, bimolecular fluorescence complementation | physical, physical association | 21044950, (Europe PMC) | 0.37 | BioGRID, IntAct |
| TLR2 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| Interacting partner | Detection method | Interaction type | PubMed IDs | Interaction Score | Source |
| APC | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| AXIN2 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| BCL10 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| BCL2L1 | Two-hybrid, two hybrid | physical, physical association | 21900206, (Europe PMC) | 0.37 | BioGRID, IntAct, MINT |
| BMPR1A | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| BRAF | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| BUB1 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| CSNK1E | Two-hybrid, two hybrid | physical, physical association | 21900206, (Europe PMC) | 0.37 | BioGRID, IntAct, MINT |
| CTNNA1 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| DCC | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| DYNC1I1 | Two-hybrid, two hybrid | physical, physical association | 21900206, (Europe PMC) | 0.37 | BioGRID, IntAct, MINT |
| ERBB2 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| FBP1 | Co-crystal Structure, Two-hybrid, split luciferase complementation, two hybrid, two hybrid array, two hybrid bait and prey pooling approach, two hybrid pooling approach, two hybrid prey pooling approach, validated two hybrid | physical, physical association | 16169070, 16189514, 21516116, 2164670, 25416956, 25910212, , (Europe PMC) | 0.91 | BioGRID, IntAct, MINT |
| FBXW7 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| FLCN | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| HDAC6 | Two-hybrid, two hybrid | physical, physical association | 21900206, (Europe PMC) | 0.37 | BioGRID, IntAct, MINT |
| MLH3 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| MSH2 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| PTK2 | Two-hybrid, two hybrid | physical, physical association | 21900206, (Europe PMC) | 0.37 | BioGRID, IntAct, MINT |
| PTPRJ | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| RB1 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| STK11 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| TLR2 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| Interacting partner | Detection method | Interaction type | PubMed IDs | Interaction Score | Source |
| APC | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| ASCC2 | Two-hybrid, two hybrid pooling approach | physical, physical association | 16169070, (Europe PMC) | 0.37 | BioGRID, IntAct |
| ASL | Two-hybrid, two hybrid | physical, physical association | 21988832, (Europe PMC) | 0.37 | BioGRID, IntAct |
| ATP5J2 | two hybrid pooling approach | physical association | 16169070, (Europe PMC) | 0.37 | IntAct |
| ATP5MF | Two-hybrid | physical | 16169070, (Europe PMC) | NA | BioGRID |
| AXIN2 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| BCL10 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| BCL2L1 | Two-hybrid, two hybrid | physical, physical association | 21900206, (Europe PMC) | 0.37 | BioGRID, IntAct, MINT |
| BIN1 | Protein-peptide, Reconstituted Complex, Two-hybrid, filter binding, peptide array, pull down, two hybrid | physical, physical association | 16275660, (Europe PMC) | 0.63 | BioGRID, IntAct |
| BMPR1A | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| BRAF | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| BUB1 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| CCDC68 | Affinity Capture-MS | physical | 26186194, 28514442, (Europe PMC) | NA | BioGRID |
| CROT | Affinity Capture-MS | physical | 28514442, (Europe PMC) | NA | BioGRID |
| CSNK1E | Two-hybrid, two hybrid | physical, physical association | 21900206, (Europe PMC) | 0.37 | BioGRID, IntAct, MINT |
| CTNNA1 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| DCC | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| DYNC1I1 | Two-hybrid, two hybrid | physical, physical association | 21900206, (Europe PMC) | 0.37 | BioGRID, IntAct, MINT |
| EPAS1 | anti bait coimmunoprecipitation, anti tag coimmunoprecipitation, pull down | direct interaction, physical association | 25043030, (Europe PMC) | 0.60 | IntAct |
| ERBB2 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| FBP1 | Co-crystal Structure, Two-hybrid, split luciferase complementation, two hybrid, two hybrid array, two hybrid bait and prey pooling approach, two hybrid pooling approach, two hybrid prey pooling approach, validated two hybrid | physical, physical association | 16169070, 16189514, 21516116, 2164670, 25416956, 25910212, , (Europe PMC) | 0.91 | BioGRID, IntAct, MINT |
| FBP2 | Affinity Capture-MS, Two-hybrid, split luciferase complementation, two hybrid array, two hybrid bait and prey pooling approach, two hybrid prey pooling approach, validated two hybrid | physical, physical association | 25416956, 25910212, 26186194, 28514442, , (Europe PMC) | 0.81 | BioGRID, IntAct |
| FBXW7 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| FLCN | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| FXR2 | Two-hybrid, two hybrid pooling approach | physical, physical association | 16189514, (Europe PMC) | 0.37 | BioGRID, IntAct |
| GIMAP2 | Affinity Capture-MS | physical | 28514442, (Europe PMC) | NA | BioGRID |
| HDAC6 | Two-hybrid, two hybrid | physical, physical association | 21900206, (Europe PMC) | 0.37 | BioGRID, IntAct, MINT |
| HIF1A | anti bait coimmunoprecipitation, anti tag coimmunoprecipitation, pull down | direct interaction, physical association | 25043030, (Europe PMC) | 0.60 | IntAct |
| HSPA8 | Two-hybrid, two hybrid pooling approach | physical, physical association | 16169070, (Europe PMC) | 0.37 | BioGRID, IntAct |
| KLRC2 | Two-hybrid, two hybrid array | physical, physical association | 21988832, (Europe PMC) | 0.37 | BioGRID, IntAct |
| LINC01667 | Affinity Capture-MS | physical | 26186194, 28514442, (Europe PMC) | NA | BioGRID |
| LNX1 | Two-hybrid, two hybrid pooling approach | physical, physical association | 16189514, (Europe PMC) | 0.37 | BioGRID, IntAct |
| MLH3 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| MSH2 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| PCNX4 | Two-hybrid | physical | 16189514, (Europe PMC) | NA | BioGRID |
| PCNX4 | two hybrid pooling approach | physical association | 16189514, (Europe PMC) | 0.37 | IntAct |
| POT1 | Two-hybrid, bimolecular fluorescence complementation | physical, physical association | 21044950, (Europe PMC) | 0.37 | BioGRID, IntAct |
| PRKN | Biochemical Activity | physical | 21209200, (Europe PMC) | NA | BioGRID |
| PTK2 | Two-hybrid, two hybrid | physical, physical association | 21900206, (Europe PMC) | 0.37 | BioGRID, IntAct, MINT |
| PTPRJ | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| RB1 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| RNF183 | Two-hybrid, two hybrid pooling approach | physical, physical association | 16189514, (Europe PMC) | 0.37 | BioGRID, IntAct |
| SAMD7 | Affinity Capture-MS | physical | 28514442, (Europe PMC) | NA | BioGRID |
| SLAMF8 | Affinity Capture-MS | physical | 28514442, (Europe PMC) | NA | BioGRID |
| ST6GALNAC5 | Affinity Capture-MS | physical | 26186194, 28514442, (Europe PMC) | NA | BioGRID |
| STK11 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| TERF1 | Two-hybrid, bimolecular fluorescence complementation | physical, physical association | 21044950, (Europe PMC) | 0.37 | BioGRID, IntAct |
| TIGD7 | Affinity Capture-MS | physical | 28514442, (Europe PMC) | NA | BioGRID |
| TLR2 | two hybrid array | physical association | 24412244, (Europe PMC) | 0.37 | IntAct, MINT |
| Phosphorylating Kinases | Phosphoresidues | Detection Method | PubMed IDs | Source |
| ULK1 | S63_HLYGIAGsTNVTGDQ, S88_LVMNMLKsSFATCVL, | NA | NA | PhosphoSitePlus, |

