
CHEBI:17407
| Name | 2-trans,6-trans-farnesyl diphosphate | 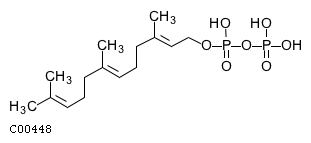 Download: mol | sdf |
| Synonyms | 0(2e,6e)-farnesyl diphosphate; 2-trans,6-trans-farnesyl diphosphate; 3,7,11-trimethyldodeca-2,6,10-trien-1-yl trihydrogen diphosphate; Farnesyl diphosphate; Farnesyl pyrophosphate; Fdp; Trans,trans-farnesyl diphosphate; | |
| Definition | The trans,trans-stereoisomer of farnesyl diphosphate. | |
| Molecular Weight (Exact mass) | 382.3261 (382.131) | |
| Molecular Formula | C15H28O7P2 | |
| SMILES | CC(C)=CCC\C(C)=C\CC\C(C)=C\COP(O)(=O)OP(O)(O)=O | |
| InChI | InChI=1S/C15H28O7P2/c1-13(2)7-5-8-14(3)9-6-10-15(4)11-12-21-24(19,20)22-23(16,17)18/h7,9,11H,5-6,8,10,12H2,1-4H3,(H,19,20)(H2,16,17,18)/b14-9+,15-11+ | |
| InChI Key | VWFJDQUYCIWHTN-YFVJMOTDSA-N | |
| Crosslinking annotations | KEGG:C00448 | 3DMET:B01245 | CAS:372-97-4 | ChEBI:17407 | ChEMBL:CHEMBL69330 | KNApSAcK:C00000907 | KNApSAcK:C00007268 | LIPIDMAPS:LMPR0103010002 | LipidBank:IIP0005 | NIKKAJI:J348.314B | PDB-CCD:FPP | PubChem:3736 | |
| Pathway ID | Pathway Name | Pathway Description (KEGG) |
| map00100 | Steroid biosynthesis | NA |
| map00900 | Terpenoid backbone biosynthesis | Terpenoids, also known as isoprenoids, are a large class of natural products consisting of isoprene (C5) units. There are two biosynthetic pathways, the mevalonate pathway [MD:M00095] and the non-mevalonate pathway or the MEP/DOXP pathway [MD:M00096], for the terpenoid building blocks: isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). The action of prenyltransferases then generates higher-order building blocks: geranyl diphosphate (GPP), farsenyl diphosphate (FPP), and geranylgeranyl diphosphate (GGPP), which are the precursors of monoterpenoids (C10), sesquiterpenoids (C15), and diterpenoids (C20), respectively. Condensation of these building blocks gives rise to the precursors of sterols (C30) and carotenoids (C40). The MEP/DOXP pathway is absent in higher animals and fungi, but in green plants the MEP/DOXP and mevalonate pathways co-exist in separate cellular compartments. The MEP/DOXP pathway, operating in the plastids, is responsible for the formation of essential oil monoterpenes and linalyl acetate, some sesquiterpenes, diterpenes, and carotenoids and phytol. The mevalonate pathway, operating in the cytosol, gives rise to triterpenes, sterols, and most sesquiterpenes. |
| map00906 | Carotenoid biosynthesis | NA |
| map00909 | Sesquiterpenoid and triterpenoid biosynthesis | Sesquiterpenoids (C15 terpenoids) are a group of terpenoids consisting of three isoprene units. They are derive from farnesyl diphosphate (FPP) and can be cyclized to produce various skeletal structures. Sesquiterpenoid biosynthesis begins with the loss of diphosphate from FPP under the action of sesquiterpene synthesis enzymes, generating an allylic cation that is highly susceptible to intramolecular attacks. Cyclization of the farnesyl cation may take place onto either of the remaining double bonds with the result that 6-, 10-, or 11-membered rings may be formed. Many sesquiterpenoids have been isolated from plants, fungi, marine organisms, and Streptomyces species. This map shows a few examples of acyclic and cyclic sesquiterpenoids. |
| map00981 | Insect hormone biosynthesis | NA |
| map00999 | Biosynthesis of secondary metabolites - unclassified | NA |
| map01060 | Biosynthesis of plant secondary metabolites | NA |
| map01062 | Biosynthesis of terpenoids and steroids | NA |
| map01066 | Biosynthesis of alkaloids derived from terpenoid and polyketide | NA |
| map01070 | Biosynthesis of plant hormones | NA |
| map01100 | Metabolic pathways | NA |
| map01110 | Biosynthesis of secondary metabolites | NA |
| map01130 | Biosynthesis of antibiotics | NA |

